Each tRNA is specifically attached to an amino acid
The charging of each tRNA with its correct amino acid is achieved by a family of enzymes known as aminoacyl-tRNA synthetases. Each enzyme is specific for one amino acid and for its corresponding tRNA. The reaction uses ATP, forming a high-energy bond between the amino acid and the tRNAs (Figure 14.11). The energy in this bond is later used in the formation of peptide bonds between amino acids in a growing polypeptide chain.
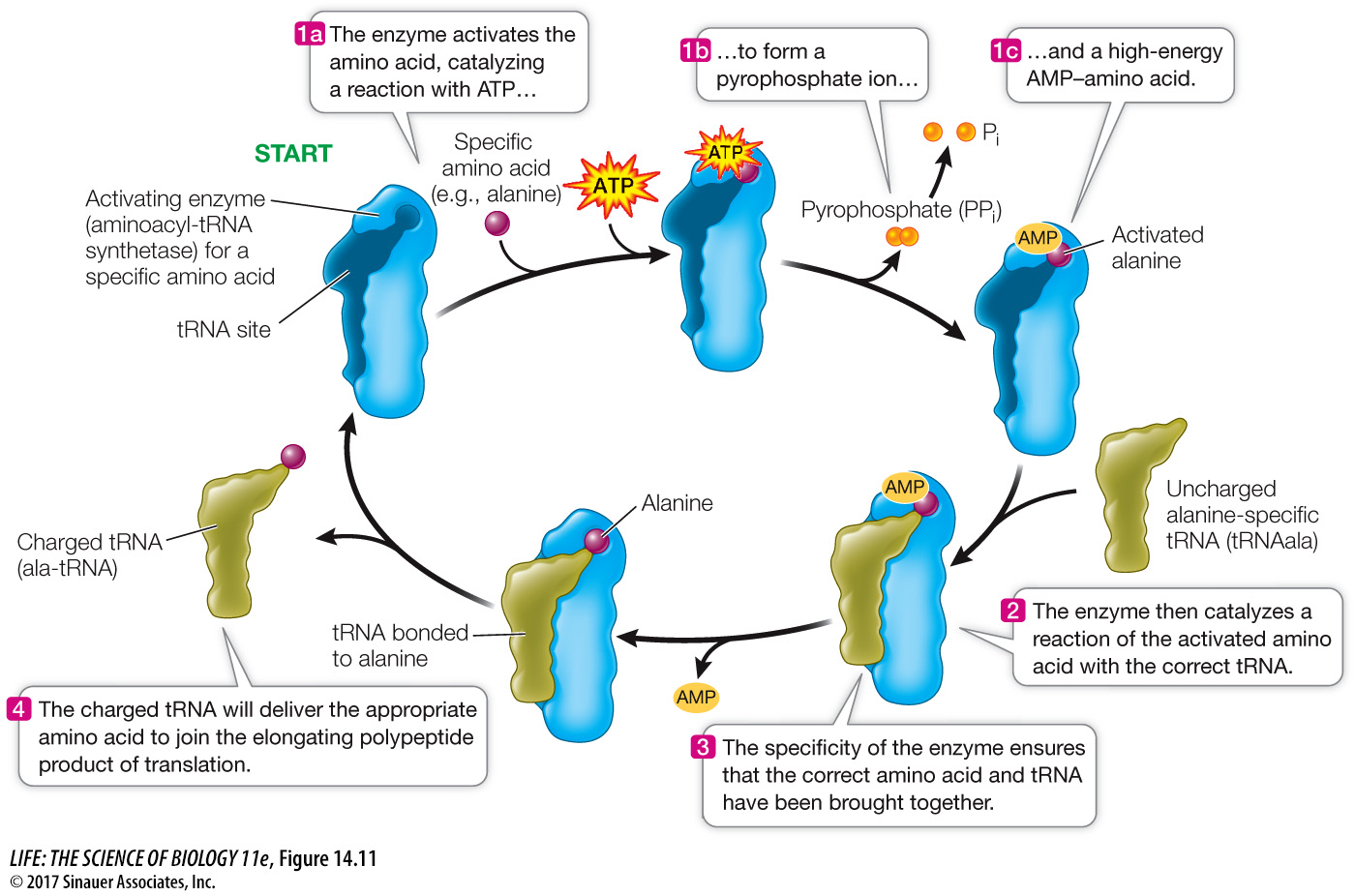
Figure 14.11 Charging a tRNA Molecule The aminoacyl-tRNA synthetase activates a specific amino acid and charges a specific tRNA with that amino acid.
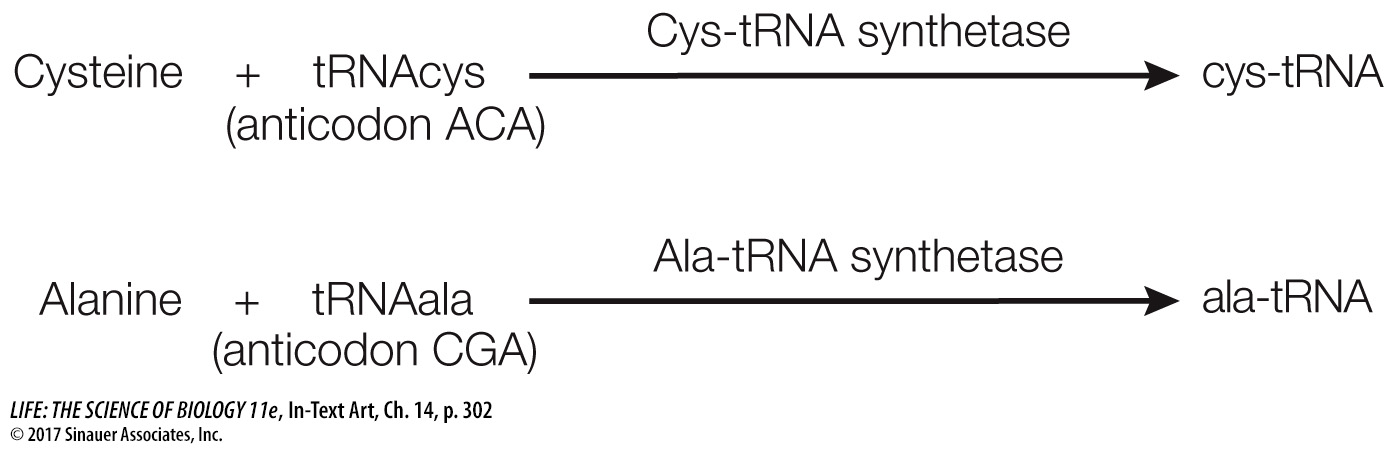
The specificity between the tRNA and its corresponding amino acid is essential. These reactions, for example, are highly specific:
A clever experiment by Seymour Benzer and his colleagues at Purdue University demonstrated the importance of this specificity. They took the cys-tRNA molecule and chemically modified the cysteine, converting it into alanine. Which component—the amino acid or the tRNA—would be recognized when this hybrid charged tRNA was put into a protein synthesizing system? The answer was the tRNA. Everywhere in the synthesized protein where cysteine was supposed to be, alanine appeared instead. The cysteine-specific tRNA had delivered its cargo (alanine) to every mRNA codon for cysteine. This experiment showed that the protein synthesis machinery recognizes the anticodon of the charged tRNA, not the amino acid attached to it.