Translation takes place in three steps
Translation is the process by which the information in mRNA (derived from DNA) is used to specify and link a specific sequence of amino acids, producing a polypeptide. Like transcription, translation occurs in three steps: initiation, elongation, and termination.
INITIATION The translation of mRNA begins with the formation of an initiation complex, which consists of a charged tRNA and a small ribosomal subunit, both bound to the mRNA (Figure 14.13).
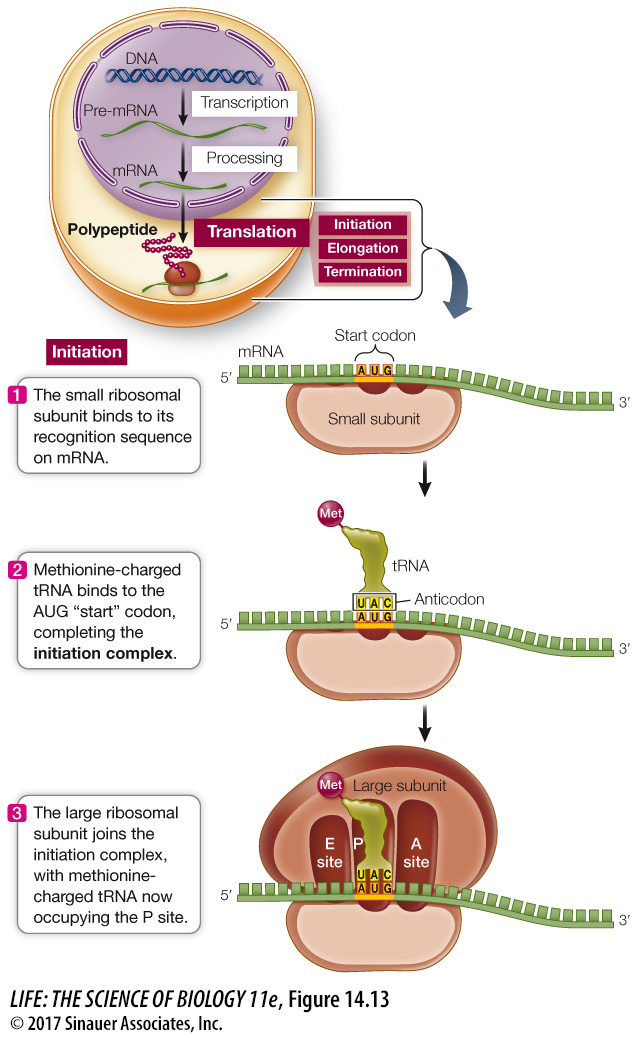
In prokaryotes, the rRNA of the small ribosomal subunit first binds to a complementary ribosome binding site (AGGAGG; known as the Shine–
mRNA 5′.........A G G A G G......(start codon).....3′
rRNA 3′.......... U C C U C C.........(P site)..........5′
Eukaryotes load the mRNA onto the ribosome somewhat differently: the small ribosomal subunit binds to the 5′ cap on the mRNA and then moves along the mRNA until it reaches the start codon.
Recall that the mRNA start codon in the genetic code is AUG (see Figure 14.5). The anticodon (UAC) of a methionine-
305
After the methionine-
ELONGATION A charged tRNA whose anticodon is complementary to the second codon of the mRNA now enters the open A site of the large ribosomal subunit (Figure 14.14). The large subunit then catalyzes two reactions:
It breaks the bond between the tRNA and its amino acid in the P site.
It catalyzes the formation of a peptide bond between the amino acid that has just been released from the P site and the one attached to the tRNA in the A site.
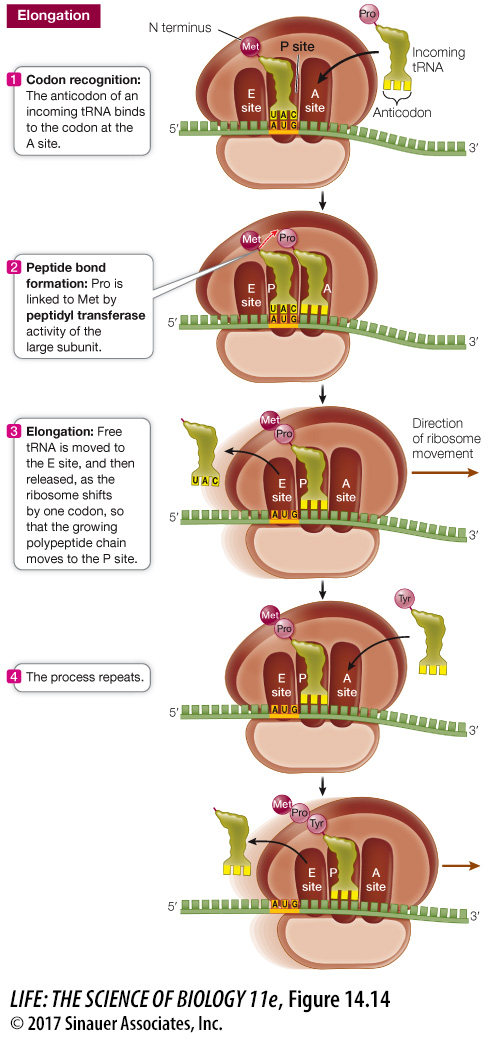
Because the large ribosomal subunit performs these two actions, it is said to have peptidyl transferase activity. In this way, methionine (the amino acid in the P site) becomes the N terminus of the new protein. The second amino acid is now bound to methionine but remains attached to its tRNA at the A site.
How does the large ribosomal subunit catalyze peptide bond formation? Harry Noller and his colleagues at the University of California at Santa Cruz did a series of experiments and found that:
if they removed almost all of the proteins from the large subunit, it still catalyzed peptide bond formation.
if the rRNA was extensively modified, peptidyl transferase activity was destroyed.
The experiment showed that rRNA is the catalyst. The purification and crystallization of ribosomes has allowed scientists to examine ribosome structure in detail, and the catalytic role of rRNA in peptidyl transferase activity has been confirmed. These findings support the hypothesis that RNA, and *catalytic RNA in particular, evolved before DNA.
After the first tRNA releases its methionine, it moves to the E site and is then dissociated from the ribosome, returning to the cytosol to become charged with another methionine. The second tRNA, now bearing a dipeptide (a two-
*connect the concepts As discussed in Key Concept 4.3, the folded, three-
306
TERMINATION The elongation cycle ends, and translation is terminated, when a stop codon—
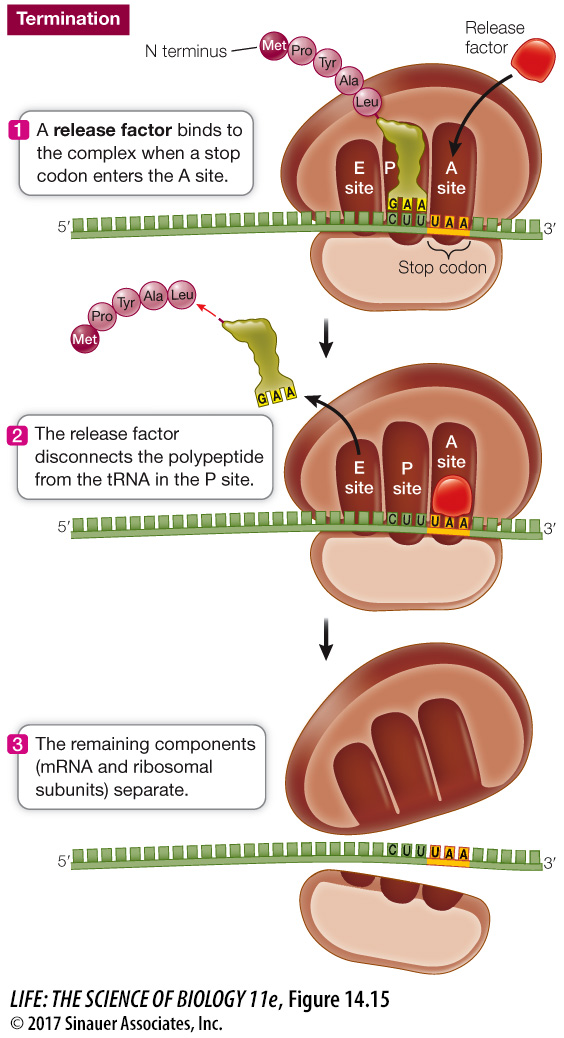
Question
Q: What happens if there is not a stop codon?
If there is no stop codon, translation continues because there are more nucleotides at the end of mRNA past the stop codon location. The protein is not properly released from the ribosome.
Table 14.3 lists the nucleic acid signals for initiation and termination of transcription and translation.
| Transcription | Translation | |
|---|---|---|
| Initiation | Promoter DNA | AUG start codon in the mRNA |
| Termination | Terminator DNA | UAA, UAG, or UGA in the mRNA |