DNA methylation occurs at the promoter and silences transcription
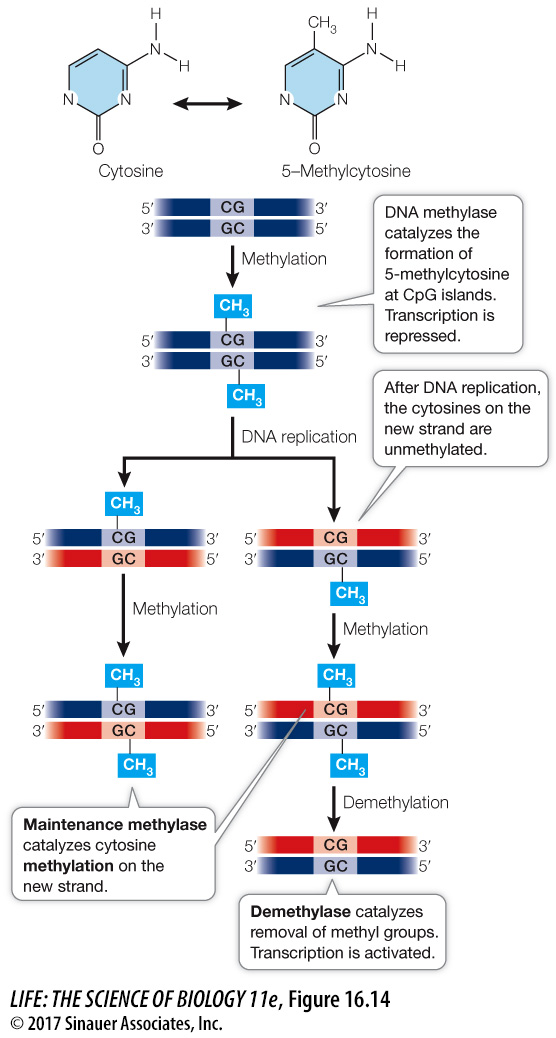
Depending on the organism, from 1 to 5 percent of cytosine residues in the organism’s DNA are chemically modified by the addition of a methyl group (—CH3) to the 5–
Question
Q: 5–
5-
This covalent change in DNA is heritable: when DNA is replicated, a maintenance methylase catalyzes the formation of 5–
What is the effect of DNA methylation? During replication and transcription, 5–
350
DNA methylation is important in development from egg to embryo. For example, when a mammalian sperm enters an egg, many genes in first the male and then the female genome become demethylated. Thus many genes that are usually inactive are expressed during early development. As the embryo develops and its cells become more specialized, genes whose products are not needed in particular cell types become methylated. These methylated genes are “silenced”; their transcription is repressed. However, unusual or abnormal events can sometimes turn silent genes back on.
For example, DNA methylation may play roles in the genesis of some cancers. In cancer cells, oncogenes get activated and promote cell division, and tumor suppressor genes (which normally inhibit cell division) are turned off (see Chapter 11). This misregulation can occur when the promoters of oncogenes become demethylated while those of tumor suppressor genes become methylated, as is the case in colorectal cancer (see Figure 15.10).