Genes can be inactivated and changed by CRISPR technology
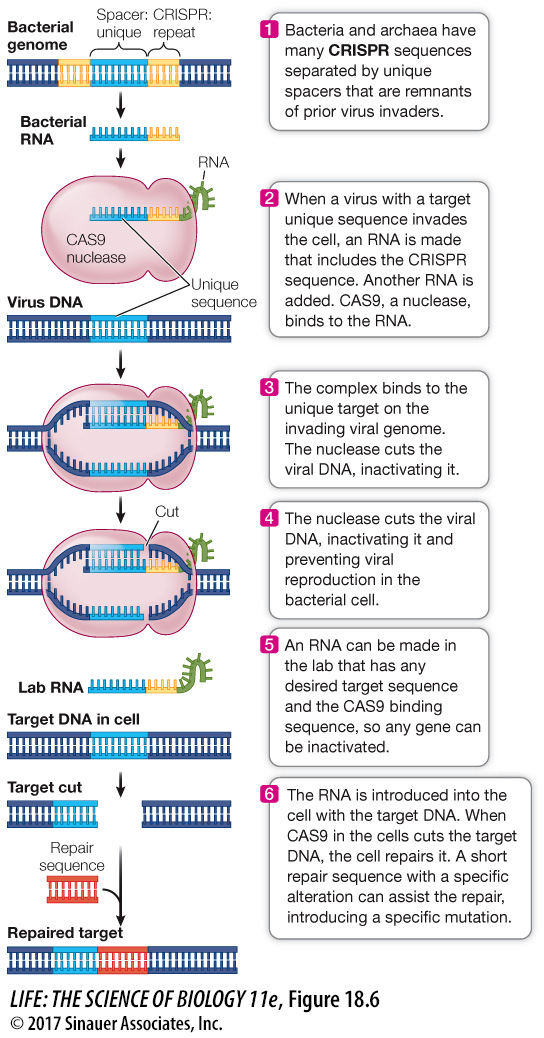
As we have just discussed, a gene can be studied by expressing it in cells where it is not normally expressed or by expressing it at higher than normal levels. Another way to study a gene is to inactivate it, so that it is not transcribed and translated into a functional protein, or to change its DNA sequence so that an altered gene product is made. CRISPR technology offers a powerful new approach for “gene editing” (adding, disrupting, or changing the sequence of specific genes) and gene regulation (Figure 18.6). As with many other tools of biotechnology, the method emulates a mechanism found in nature, one that some bacteria and many archaea use to defend themselves against viral infections. The genomes of these species have many short (24–
Because the spacer sequence is complementary to part of the viral genome, it attaches to it by base pairing.
389
The complex binds a protein called CAS9, which is a nuclease that cuts the invading viral DNA, destroying its ability to produce new viruses and thereby protecting the host cell.
research tools
Figure 18.6 Inactivating or Mutating a Gene by CRISPR A molecular system used by bacteria to combat invading viruses has been adapted to inactivate or mutate any gene.
Activity 18.1 CRISPR Simulation
www.life11e.com/
Therefore any cell that was once infected by a virus has the “memory” of that infection encoded in its genome, and those of all cells that are produced from it.
Once this natural immune system was described, it occurred to Emmanuelle Charpentier at Umeå University in Sweden and Jennifer Doudna at the University of California, Berkeley that it might be adapted to cut any DNA at a specific location: all that was needed was the RNA complementary to the target (a “guide” RNA) and the CAS9 enzyme. Tens of thousands of unique guide RNAs are now available commercially, each targeted to a specific gene in a specific organism, prokaryotic or eukaryotic, making possible a diversity of studies of inactivated genes and exploration of a multitude of “What if?” questions.
The simplicity of CRISPR technology to inactivate genes holds great promise. To give just one early example, a fungal disease called powdery mildew can devastate a wheat crop. Many wheat varieties have a gene whose expression inhibits wheat’s natural defenses against this disease, so the only way to control the disease is through heavy application of fungicides. Scientists in China used CRISPR technology to inactivate this gene, so that natural resistance is favored.
CRISPR technology can be used to create specific mutations. When CAS9 cuts target DNA in a living cell, the cell attempts to repair the damage (see Figure 13.18). Often this repair is imperfect, so that the rejoined DNA has a mutation. Biologists can specify the mutation by synthesizing a short DNA sequence complementary to the region at the break so that the new sequence will have a specific alteration.
Perhaps more dramatic than the ability to induce mutations is the possibility of correcting mutations, particularly those that cause disease. For example, the disease cystic fibrosis is caused by mutation of a membrane transport protein. Scientists in the Netherlands have used CRISPR to correct the mutation in cells from patients with this disease. While this is not a cure (the corrected gene would have to function in many tissues), the possibility arises that the mutated gene could be corrected in germ line cells, such as those of an early embryo. However, the ease with which the human germ line genome can be altered using CRISPR technology has elicited ethical and legal concerns. In some countries altering the germ line in a human is illegal.