Pluripotent stem cells can be obtained in two ways
As stated earlier, totipotent stem cells that can differentiate into any cell type are found only in very early embryos. In both mice and humans, a slightly later embryonic stage is a hollow sphere of cells called a blastocyst (see Figure 43.4). A group of cells within the blastocyst is pluripotent: they can differentiate into most cell types but cannot give rise to a complete organism. In mice, these embryonic stem cells (ESCs) can be removed from the blastocyst and grown in laboratory culture almost indefinitely, given the right conditions. When cultured mouse ESCs are injected back into another mouse blastocyst, the stem cells mix with the resident cells and differentiate to form all the cell types in the mouse, which indicates that ESCs retain their developmental potential while growing in the laboratory.
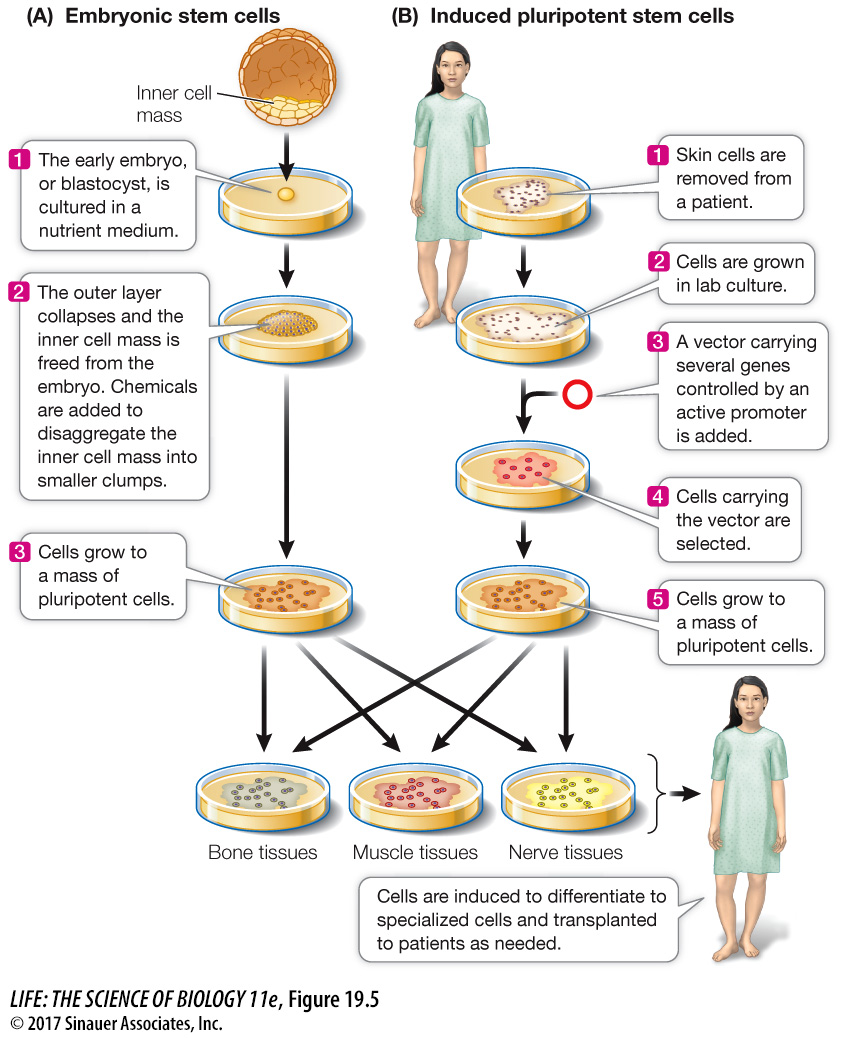
ESCs growing in the laboratory can also be induced to differentiate in a particular way if the right signal is provided (Figure 19.5A). For example, treatment of mouse ESCs with a derivative of vitamin A causes them to form neurons, whereas other growth factors induce them to form blood cells. Such experiments demonstrate both the cells’ developmental potential and the roles of environmental signals. This finding raises the possibility of using ESC cultures as sources of differentiated cells to repair specific tissues, such as a damaged pancreas in diabetes, or a brain that malfunctions in Parkinson’s disease.
Animation 19.1 Embryonic Stem Cells
www.life11e.com/
ESCs can be harvested from human embryos conceived by in vitro (“under glass”—in the laboratory) fertilization, with the consent of the parents that conceived them. Since more than one embryo is usually conceived in this procedure, there is great interest in clinical studies of ESCs from embryos not used for reproduction. While there is substantial promise in the possibility of using ESCs from human embryos as sources of tissues for transplantation into patients with tissue damage, serious concerns must first be addressed, including the ethical issue of whether human embryos should be destroyed for this (or any) purpose, and the possibility that stem cells, and tissues derived from them, might provoke an immune response in a recipient (see Chapter 41). Shinya Yamanaka and coworkers at Kyoto University in Japan have developed a way to produce pluripotent stem cells that gets around these two problems (Figure 19.5B). Instead of extracting ESCs from blastocysts, they make pluripotent stem cells from skin cells. They developed this method systematically:
406
First they used microarrays (see Figure 18.8) to compare the genes expressed in ESCs with non-
stem cells. They found several genes that were uniquely expressed at high levels in ESCs. These genes were believed to be essential to the undifferentiated state and function of stem cells. Next they isolated the genes, coupled them to highly expressing promoters, and inserted them into skin cells (see Key Concept 18.5). They found that the skin cells now expressed the newly added genes at high levels.
Finally, they showed that the altered skin cells were pluripotent and could be induced to differentiate into many tissues. They called these cells induced pluripotent stem cells (iPS cells).
Because the iPS cells can be made from skin cells of the individual who is to be treated, an immune response may be avoided. Such cells have already been used for cell therapy in animals for diseases similar to human Parkinson’s disease (a brain disorder), diabetes, and sickle-