recap
568
26.4 recap
Protists have many effects, both positive and negative, on their environment. Some species are important primary producers, many are endosymbionts, and some are pathogens. Protists are among the most important producers of fossil fuels, and they are important components of sedimentary rocks.
learning outcomes
You should be able to:
Describe the complex life cycle of a parasite that involves multiple hosts.
Summarize the consequences of overproduction of some diatoms and dinoflagellates.
Describe beneficial and harmful interactions among species that involve protists.
Give examples of protists that are important to humans.
Question 1
What is the role of female Anopheles mosquitoes in the transmission of malaria?
The female mosquitoes transmit the Plasmodium parasites to vertebrate hosts. The mosquitoes take up Plasmodium along with blood from an infected host and transmit the parasite to new, previously uninfected hosts when they feed on the blood of the new hosts.
Question 2
Explain the roles of dinoflagellates in the two very different phenomena of coral bleaching and red tides.
Red tides are massive blooms of free-
Question 3
What are two ways in which diatoms are important to human society?
Diatoms are important as primary producers in many ecosystems. Of more direct importance to humans, fossilized oils from diatoms are the primary source of petroleum and natural gas. In addition, the remains of diatom skeletons produce diatomaceous earth, which is used for insulation, filtration, polishing, and as an insecticide.
The next six chapters will explore the major evolutionary radiations of multicellular eukaryotes, along with the protist ancestors from which they arose. Chapters 27 and 28 will describe the origin and diversification of plants, Chapter 29 will present the fungi, and Chapters 30–32 will provide a brief overview of the animals.
569
investigating life
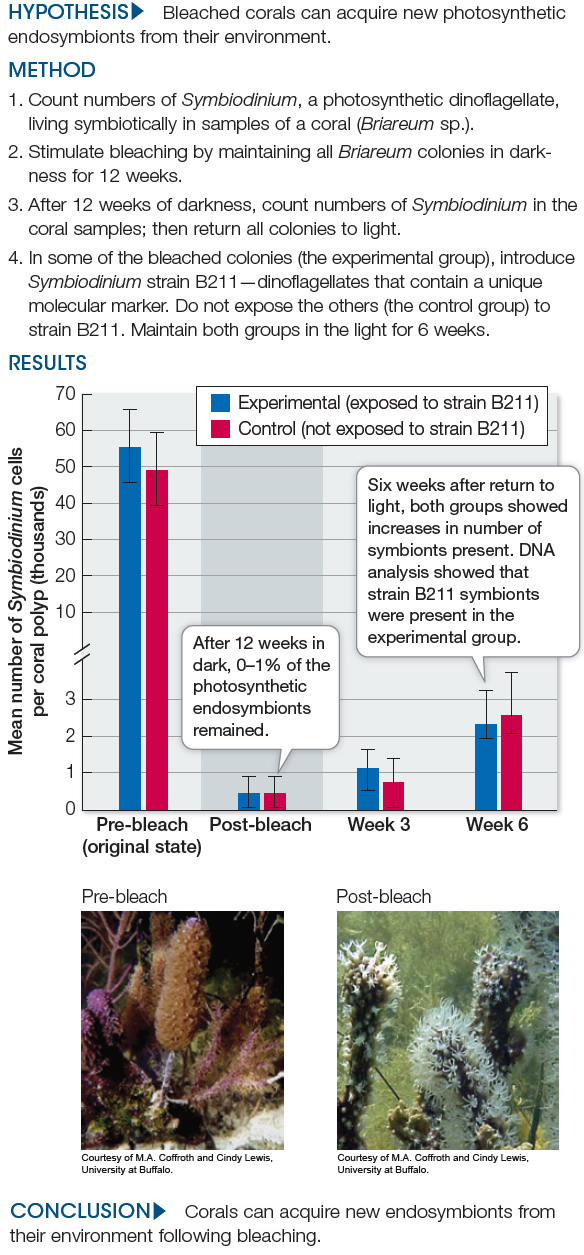
Can Corals Reacquire Dinoflagellate Endosymbionts Lost to Bleaching?
experiment
Original Paper: Lewis, C. L. and M. A. Coffroth. 2004. The acquisition of exogenous algal symbionts by an octocoral after bleaching. Science 304: 1490–
Some corals lose their chief nutritional source when their photosynthetic endosymbionts die, often as a result of changing environmental conditions. This experiment by Cynthia Lewis and Mary Alice Coffroth investigated the ability of corals to acquire new endosymbionts after bleaching.
work with the data
The data in the table below come from DNA analyses of Symbiodinium strains found in the experimental and control colonies of corals (Briareum) before and after bleaching. Symbiodinium strain B211 (which was not present before bleaching) was introduced to the experimental colonies after bleaching. Use these data to answer the questions below.
| Symbiodinium strain present (% of colonies) | ||||
|---|---|---|---|---|
| Non- |
B211 | Nonea | Colony died | |
| Experimental colonies (strain B211 added) | ||||
| Pre- |
100 | 0 | 0 | 0 |
| Post- |
58 | 0 | 42 | 0 |
| Week 3 | 0 | 92 | 0 | 8 |
| Week 6 | 8 | 58 | 8 | 25 |
| Control colonies (no strain B211) | ||||
| Pre- |
100 | 0 | 0 | 0 |
| Post- |
67 | 0 | 33 | 0 |
| Week 3 | 67 | 0 | 33 | 0 |
| Week 6 | 67 | 0 | 17 | 17 |
aColonies remained alive but no Symbiodinium were detected.
QUESTIONS
Question 1
Are new strains of Symbiodinium taken up only by coral colonies that have lost all their original endosymbionts?
No. The new strain (B211 in this experiment) was taken up by 92 percent of the coral colonies after bleaching (and by 100% of the surviving colonies), even though at least 58 percent of the experimental colonies retained some of their original symbionts after bleaching. This shows that bleached coral colonies are likely to take up strains of Symbiodinium that are available in the environment, even if some of their original symbionts survive the bleaching.
Question 2
Does the acquisition of a new Symbiodinium strain always result in survival of a recovering Briareum colony?
No. Between week 3 and week 6 after bleaching, an additional 17 percent of the experimental colonies died despite having acquired new strains of Symbiodinium.
Question 3
In week 3, only strain B211 was detected in the experimental colonies, but in week 6, non-
This observation suggests that the detection assays for Symbiodinium are not sensitive enough to detect very small levels of the symbionts. Presumably, the non-
A similar work with the data exercise may be assigned in LaunchPad.