Water potential differences govern the direction of water movement
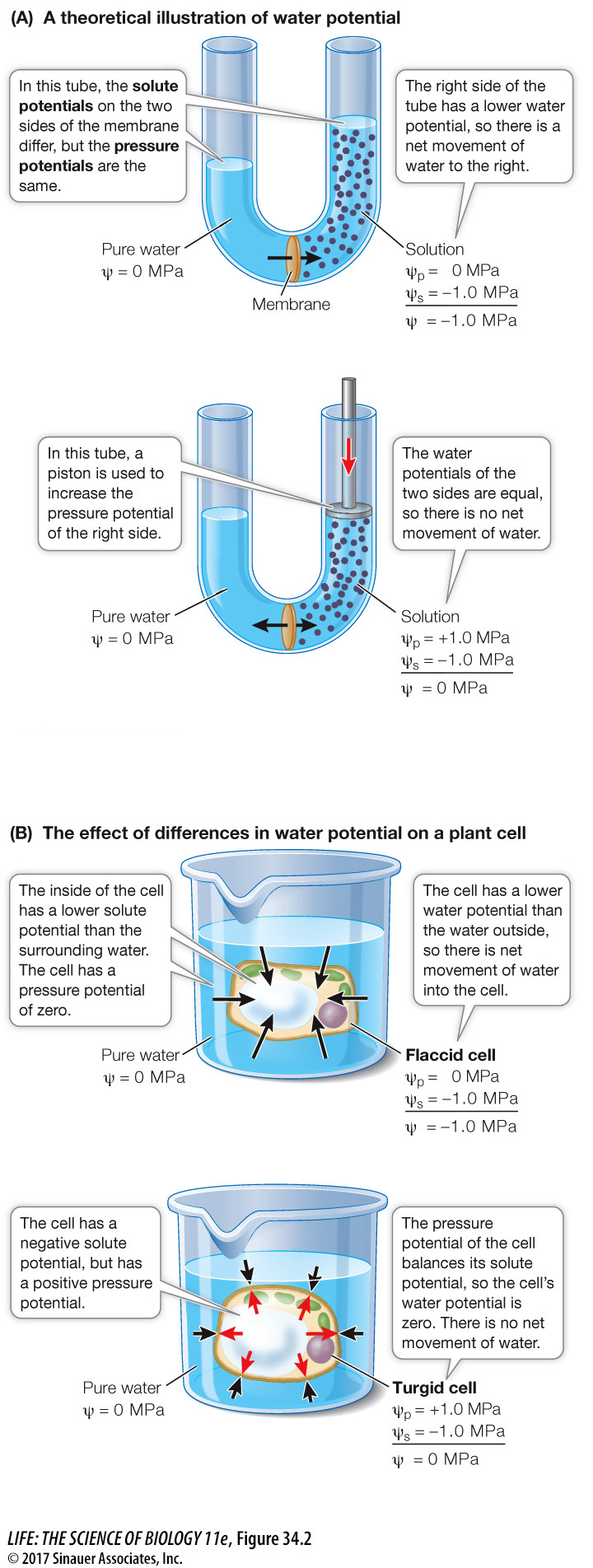
Water moves into and out of plant cells. The direction of water movement is determined by water potential (Ψ, psi), defined as the tendency of water molecules to move in response to forces such as pressure or solute concentration gradients. In the case of plant roots in soil water, whenever water moves across a selectively permeable membrane by *osmosis, it moves toward the region of lower (more negative) water potential (Figure 34.2A, top). We measure water potential in megapascals (MPa), a unit of pressure. Atmospheric pressure, 1 atmosphere, is about 0.1 MPa, or 14.7 pounds per square inch; a typical pressure in an automobile tire is about 0.2 MPa.
*connect the concepts The key to water movement is osmosis. You can review the process of osmosis and the role of the cell membrane in Key Concept 6.3.
Activity 34.1 Water Uptake in Plants
www.life11e.com/
Water potential has two major components:
Solute potential (Ψs): As solutes are added to pure water, the concentration of free water is reduced, the tendency to take up water increases, and water potential decreases.
Pressure potential (Ψp): As plant cells take up water, they tend to swell. However, the presence of the cell wall provides resistance to swelling (see Figure 6.10). The result is an increase in pressure inside the cell (turgor pressure), which decreases the tendency of the cell to take up more water (increases the water potential) (Figure 34.2A, bottom).
A solution’s water potential is the sum of its (usually negative) solute potential (Ψs) and its (usually positive) pressure potential (Ψp):
Ψ = Ψs + Ψp
By definition, the solute potential of pure water is zero (no solutes); because added solutes decrease water potential, solute potential is usually negative. Pressure potential is defined as zero when it equals atmospheric pressure. Pressure potential less than atmospheric pressure is negative; pressure potential greater than atmospheric pressure is positive.
In a plant cell immersed in pure water (Figure 34.2B), turgor pressure is comparable to the pressure potential exerted by the piston in Figure 34.2A. Water enters the cell by osmosis until the pressure potential exactly balances the solute potential and the water potential is zero. At this point the cell is turgid—that is, it has a significantly positive pressure potential. Plant cells are surrounded by water with dissolved solutes rather than pure water, and their water potential is dependent on the water potential in the soil. But because turgid cells have a positive pressure potential, there is no net movement of water into them. The physical structures of many plants are maintained by the positive pressure potential of the water in their cells. If the pressure potential drops (for example, if the plant does not have enough water), the plant wilts (Figure 34.3).

Question
Q: Sometimes a plant will wilt even if it is adequately watered because of an excess of fertilizer (dissolved ions in the soil). Why?
A hypertonic environment (a higher concentration of solutes outside than inside root cells) will result in osmosis. Plants wilt when water leaves root cells, reducing turgor pressure.
In living plant tissues, the movement of water from cell to cell follows a gradient of water potential. But over longer distances, in unobstructed tubes such as xylem vessels, the flow of water and dissolved solutes is driven by a gradient of pressure potential, not a gradient of water potential. The movement of a solution from a region of higher pressure potential to a region of lower pressure potential is called bulk flow.