Water and ions move across the root cell’s cell membrane
737
Now that you’ve seen how water can move into plant cells by osmosis, what about ions? And what is the role of the cell membrane? The movement of water and mineral ions across a root cell membrane can be impeded for two reasons:
The interior of the membrane is hydrophobic, whereas water and mineral ions are polar.
Some mineral ions must be moved against their concentration gradients.
However, as you saw in Chapter 6, membrane proteins assist with the movement of materials across membranes:
Aquaporins. Aquaporins (see Key Concept 6.3) are located in both the cell membrane and the tonoplast (vacuolar membrane) of a plant cell. Aquaporins allow water to diffuse rapidly across these membranes. The number of aquaporins in a particular cell depends on that cell’s need to obtain and retain water, and can vary with environmental conditions. The permeability of some aquaporins also can be regulated. Alterations in aquaporin abundance and permeability change the rate of osmosis across the membrane. Note that water movement through aquaporins is always passive: from a region of higher water potential to one of lower water potential.
738
Ion channels and pumps. When the concentration of a charged ion in the soil is greater than that in the plant, transport proteins can move the ions into the plant by facilitated diffusion, which is a passive process (see Key Concept 6.3). The concentrations of most ions in the soil solution, however, are lower than those inside the plant. In these cases the plant must actively take up ions against their concentration gradients—
a process that requires energy (see Key Concept 6.4).
Electric charge differences also play a role in the uptake of mineral ions. For example, a negatively charged ion that moves into a negatively charged compartment is moving against an electrical gradient, and this requires energy. Concentration and electrical gradients combine to form an *electrochemical gradient. Uptake against an electrochemical gradient involves active transport, which requires energy and specific transport proteins.
*connect the concepts Electrochemical gradients are important in many biological systems. Learn more about electrochemical gradients, and their role in the animal nervous system, in Key Concept 44.2.
Unlike animals, plants do not have a sodium–
An electrical gradient is created, with the region outside the cell more positively charged than the inside.
A proton concentration gradient develops, with more protons outside the cell than inside.
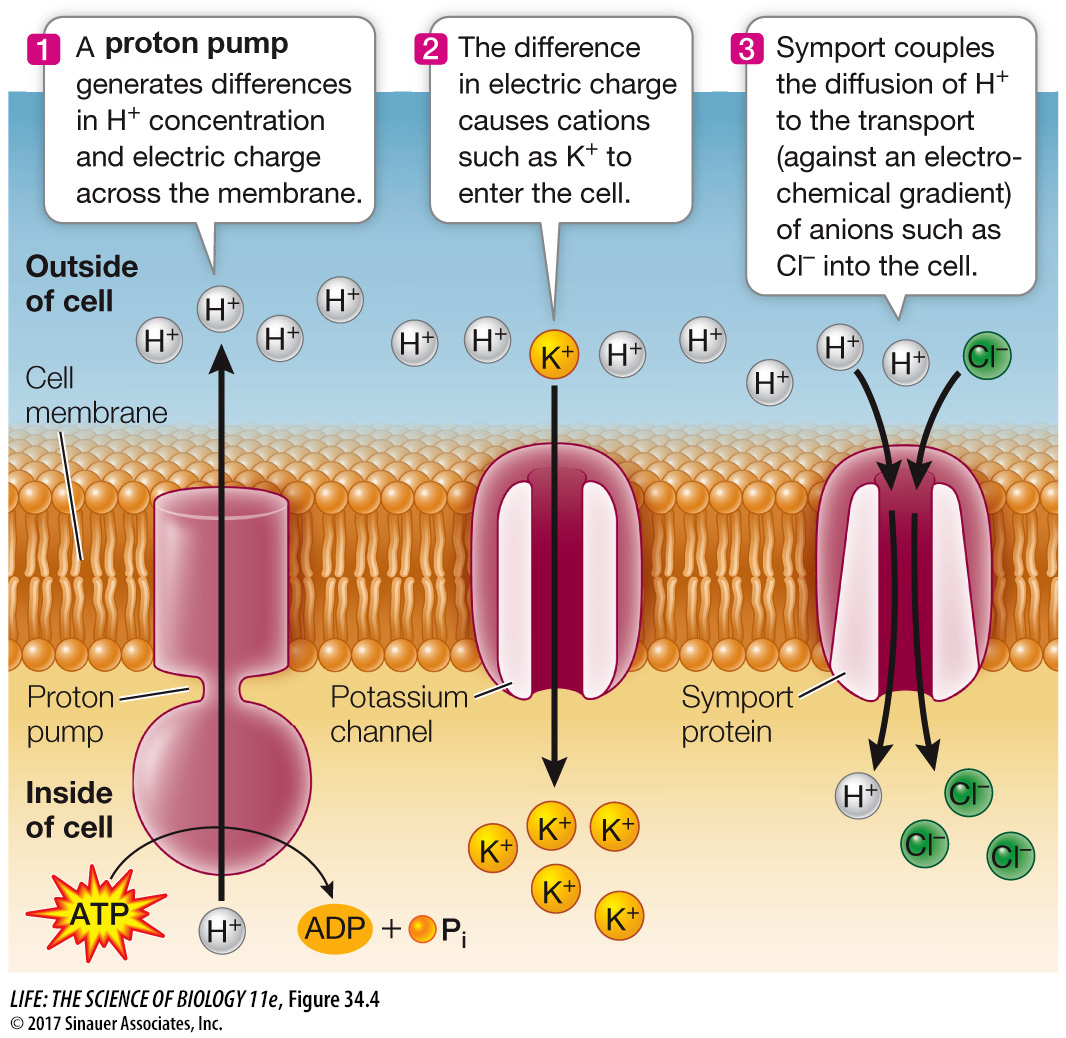
Both the electrical gradient and the concentration gradient assist with the movement of other ions into the cell. Because the inside of the cell is more negative than the outside, cations (positively charged ions) such as potassium (K+) can move into the cell by facilitated diffusion through specific membrane channels (Figure 34.4, Step 2). In addition, the proton concentration gradient can be harnessed to drive secondary active transport, in which anions (negatively charged ions) such as chloride (Cl–) are moved into the cell. These ions can move against the electrochemical gradient because symport proteins couple their movement with that of H+ (Figure 34.4, Step 3).