Soils are the source of plant nutrition
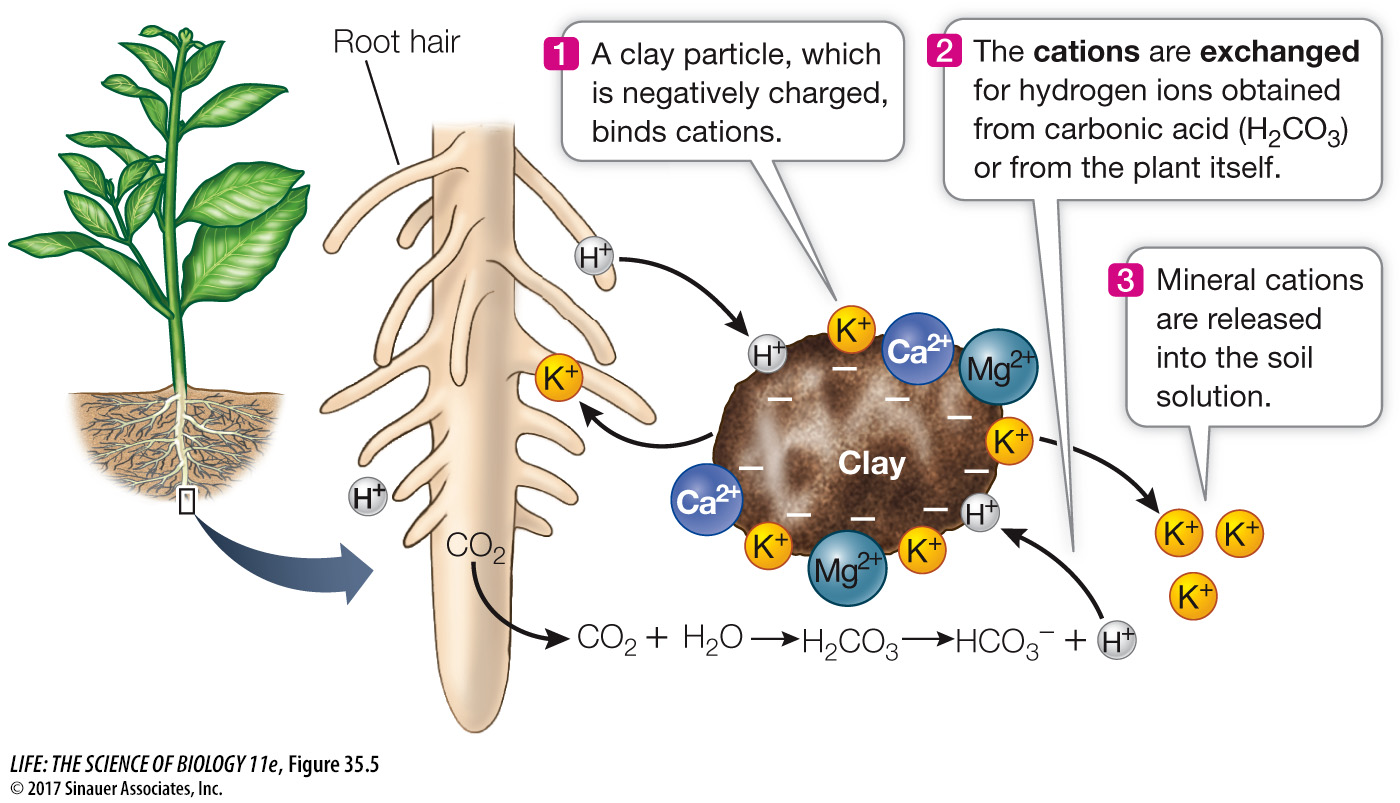
Humus and clay particles often carry negative charges. These particles form ionic attractions (see Key Concept 2.2) with the positively charged ions (cations) of many minerals that are important for plant nutrition, such as potassium (K+), magnesium (Mg2+), and calcium (Ca2+). To become available to plants or other organisms, these cations must be detached from the clay particles. How can this breakage of ionic attraction happen?
Recall that the root surface is covered with root hair cells (see Figure 33.10). Transporters in the cell membranes of these cells actively pump protons (H+) out of the cell. In addition, cellular respiration in the roots releases CO2, which dissolves in the soil water and reacts with it to form carbonic acid. This acid ionizes to form bicarbonate and free protons:
CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3–
Proton-
Question
Q: Do negatively charged ions behave differently?
Negatively charged ions (anions) can bind to certain soil particles that are positively charged and undergo ion exchange. But this does not occur at the pH of typical soils. For most soils with negatively charged particles, anions can leach out of the soil rapidly unless taken up by the plant roots.
Some soil particles, such as ones containing oxides of iron or aluminium, are positively charged under acid conditions and can exchange anions (Cl–, for example) in a process similar to cation exchange. However, soil pH is rarely low enough for anion exchange to occur. As a result, important anions such as nitrate (NO3–) and sulfate (SO42–
As you’ve seen, soil fertility is affected by soil pH. The H+ concentration affects the binding of cations and anions to soil particles, and can affect the solubility of other nutrients, such as iron. In addition, soil pH affects the absorption of nutrients by plant roots. The pH level of a soil depends on its mineral and organic contents and can be altered by various factors, including rainfall, weathering, plant growth, and fertilizer applications. The optimal soil pH for most plants is in the range 6–