Auxin plays a role in differential plant growth
Auxin was first discovered for its role in the bending of plants toward light. Subsequent research has shown that auxin is involved in many other aspects of plant growth and development.
IDENTIFYING AUXIN AND ITS TRANSPORT Auxin (from the Latin “to increase”) was discovered in the context of phototropism: a response to light in which plant stems bend toward a light source. This was a familiar observation by biologists and home gardeners when the ever-
Animation 36.1 Tropisms
www.life11e.com/
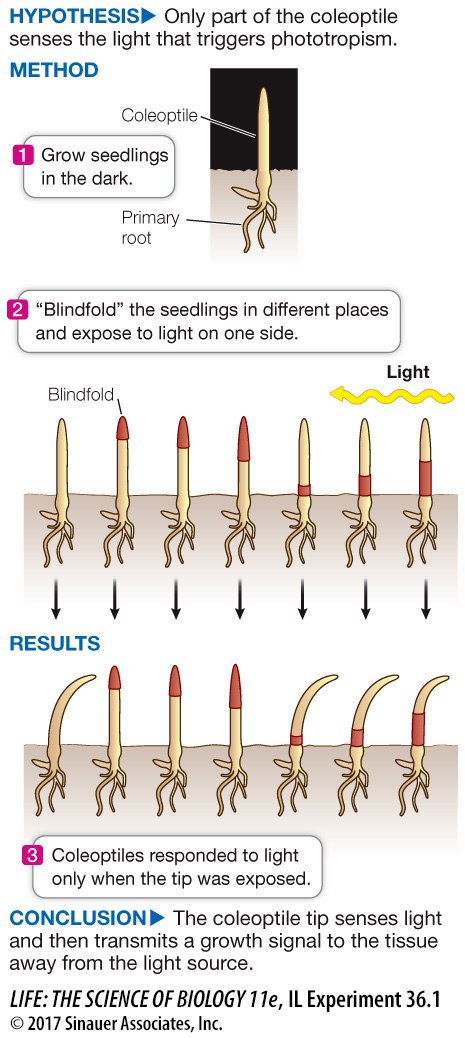
The Darwins worked with canarygrass (Phalaris canariensis) seedlings grown in the dark. While underground, a young grass seedling is protected by a coleoptile (see Figure 36.1). The coleoptiles of grasses are phototropic—

To find the light-
772
investigating life
The Darwins’ Phototropism Experiment
experiment
Original Paper: Darwin, C. R. assisted by F. Darwin. 1880. The Power of Movement in Plants. Chapter IX: Sensitiveness of plants to light: Its transmitted effects. London, John Murray.
Charles Darwin and his son Francis wanted to know how plants bend toward the light. They grew canarygrass seedlings (coleoptiles) in the dark. To discover what part of the coleoptile responds to light, they covered up (“blindfolded”) different regions of each coleoptile and then exposed the seedlings to light from one side. The Darwins discovered that the tip of the seedling senses the light and that growth occurs below the tip. Their observations led them to hypothesize the existence of a growth-
work with the data
Fascinated by experiments showing that light induces plants to bend toward it and that this is due to increased growth on the “dark side” of the stem, Charles Darwin and his son Francis performed additional experiments on canarygrass (Phalaris canariensis) seedlings grown in the dark to learn more about how the seed germinates in the soil. Their conclusion that the light-
QUESTIONS
Question 1
The figure shows a drawing by the Darwins of the bending of coleoptiles after 8 hours of light exposure. From which direction was the light shone?
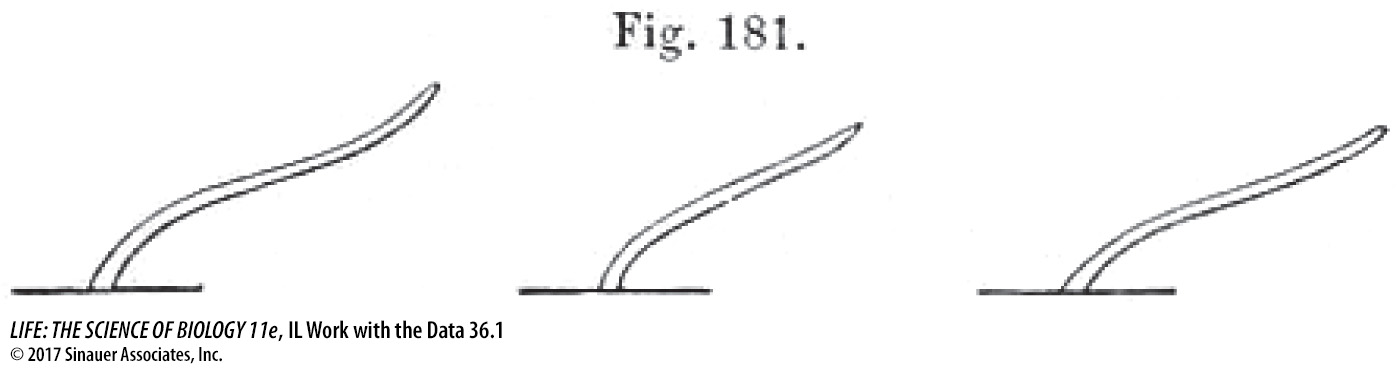
Light was shone from the right of the observer.
Question 2
The Darwins reported:
“Seven cotyledons [Note: The Darwins used the term “cotyledon” for what is now called a coleoptile.] had their tips cut off for lengths varying between 0.1 and 0.16 of an inch, and these, when left exposed all day to a lateral light, remained upright. In another set of 7 cotyledons, the tips were cut off for a length of only about 0.05 of an inch (1.27 mm) and these became bowed towards a lateral light, but not nearly so much as the many other seedlings in the same pots.”
What do these data indicate about the possible role of the tip and about the possibility that injury in cutting blocks the bending response?
These data indicate that the tip is necessary for bending to light (if the tip was cut off, the plant did not bend). The fact that cutting the very apex (1.27 mm) of the tip allowed some bending indicates that there was not significant injury and the plant was still functional.
Question 3
The Darwins described their further experiments:
“The summits of nine cotyledons, differing somewhat in height, were enclosed for rather less than half their lengths in uncoloured or transparent tubes; and these were then exposed before a south-
What do these data indicate about the role of the tip? Can you explain why there was slight bending in six of the coleoptiles that were covered with painted tubes?
The data indicate that exposure of the tip to light is necessary for the bending response. The six coleoptiles that bent slightly even though they were covered possibly were ineffectually covered—
A similar work with the data exercise may be assigned in LaunchPad.
773
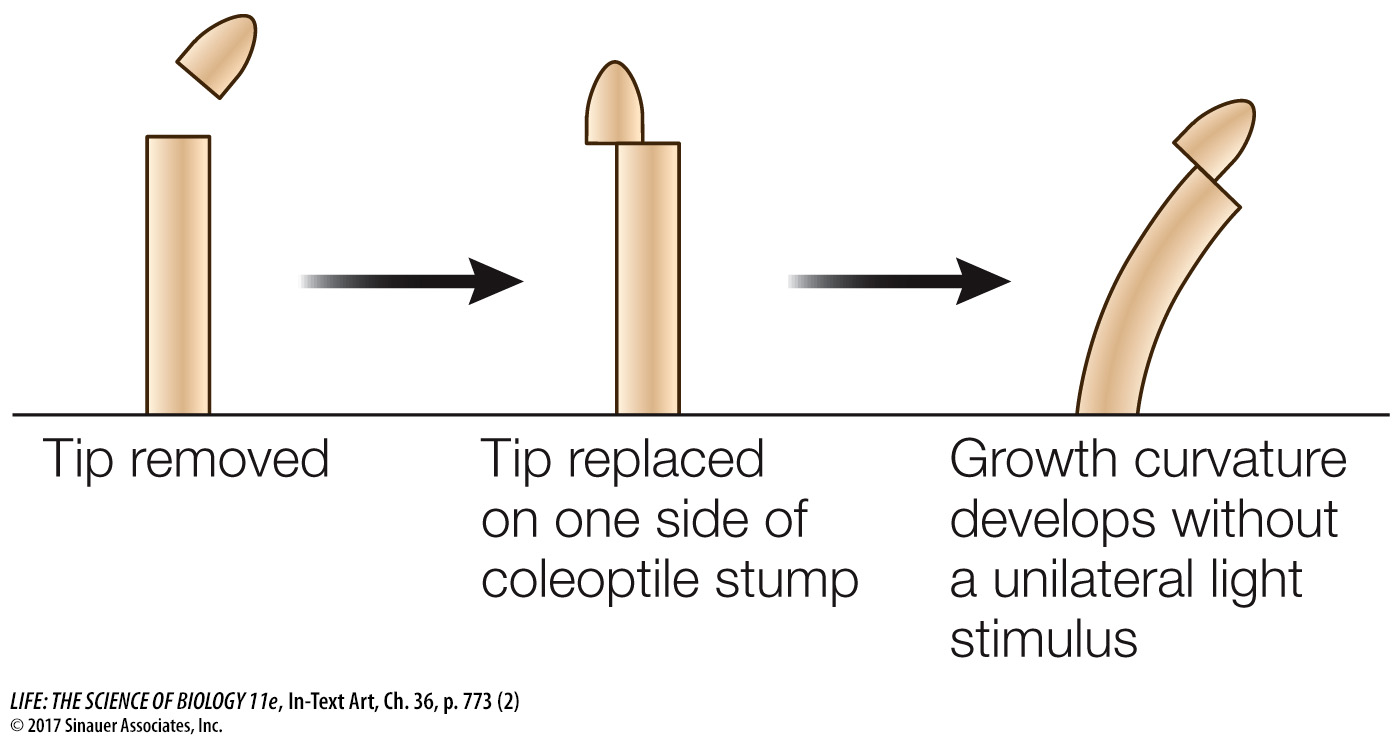
Others showed that placing the coleoptile tip (the source of the growth signal) on a decapitated coleoptile led to bending, even when a block of gelatin separated the tip and coleoptile.
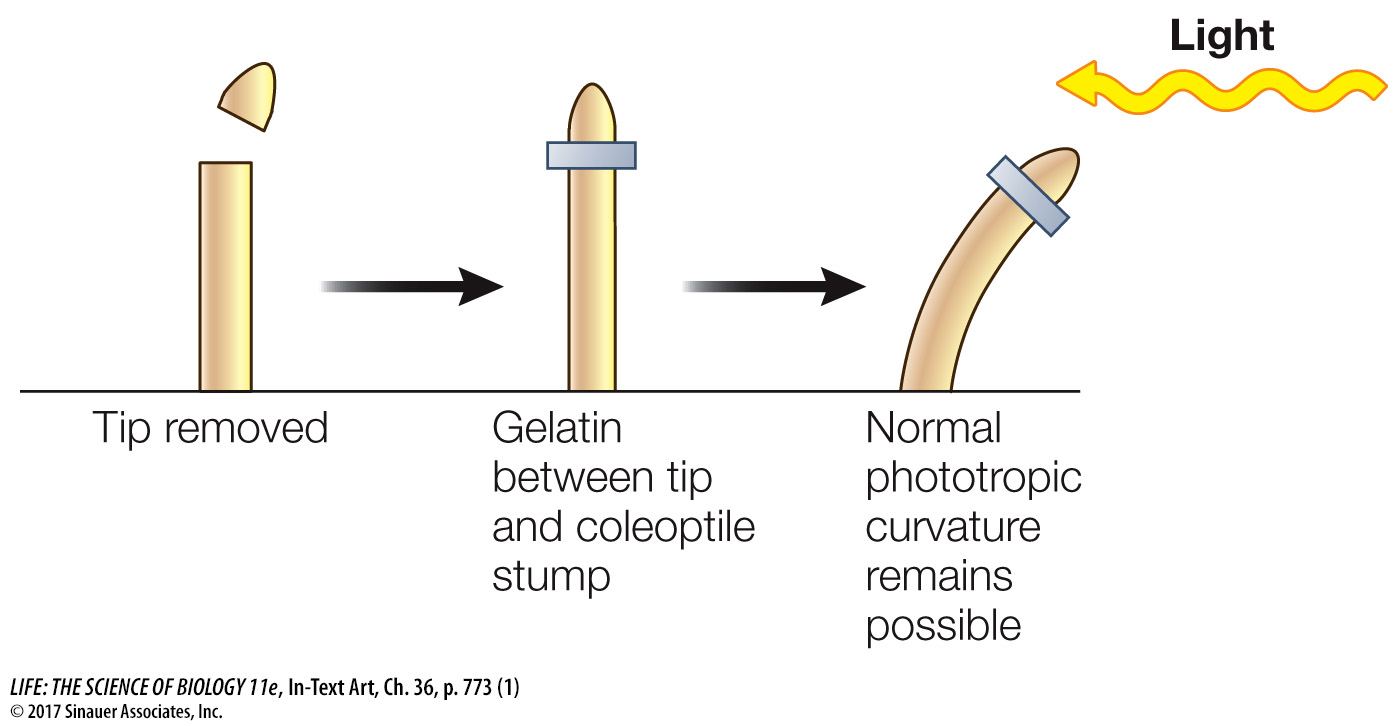
In these experiments, the soluble growth signal moved from the coleoptile tip into the gelatin and then down into the decapitated coleoptile; later the growth signal was isolated from such gelatin blocks and identified as indole-
Animation 36.2 Went’s Experiment
www.life11e.com/
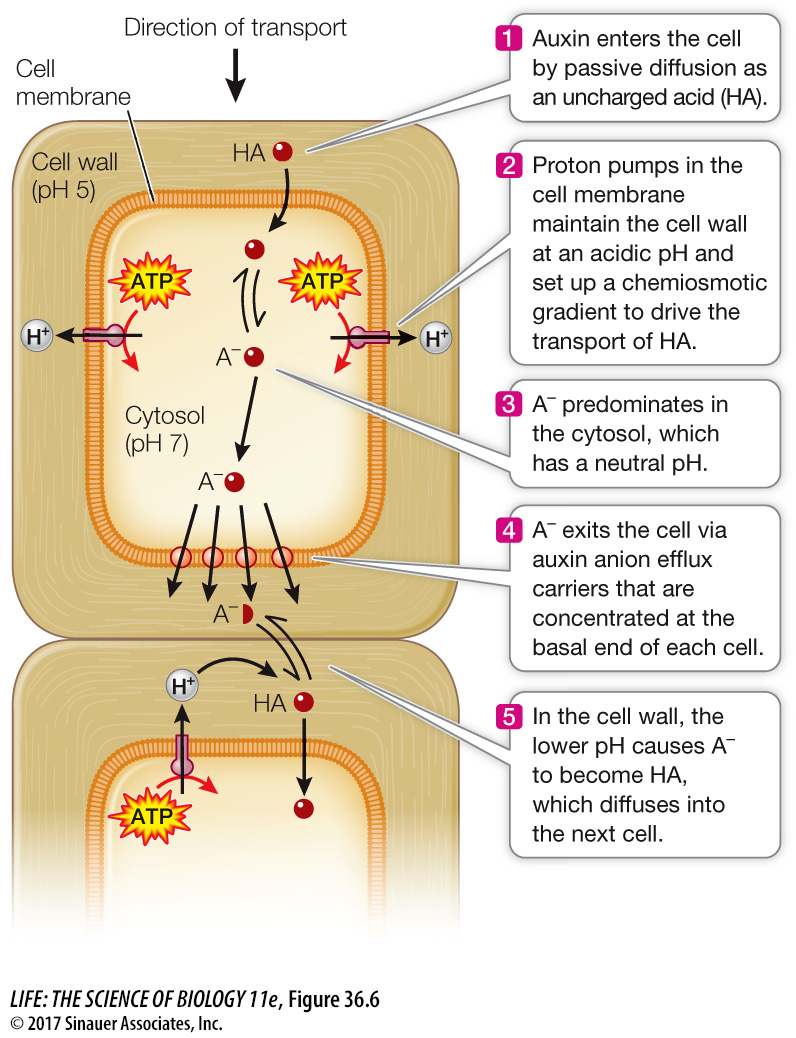
MECHANISM OF AUXIN TRANSPORT The movement of auxin down a coleoptile is an example of polar (apical-
Diffusion across a cell membrane. Polar molecules (in the chemical sense) diffuse across cell membranes less readily than nonpolar molecules (see Key Concept 6.3).
Membrane protein asymmetry. Active transport carriers (see Key Concept 6.4) for auxin are located only in the portion of the cell membrane at the basal (bottom) end of the cell.
Proton pumping/chemiosmosis. A proton pump (see Figure 34.4) moves H+ from the cytoplasm to the cell wall, thereby increasing the intracellular pH and decreasing the pH in the cell wall. Proton pumping also sets up an electrochemical gradient (see Key Concept 9.3), which provides potential energy to drive the transport of auxin by the carriers mentioned above.
Ionization of a weak acid. The main form of auxin, indole-
3- acetic acid, is a weak acid (see Key Concept 2.4): A– + H+ ⇌ HA
When the pH is low, this reaction is driven to the right, and HA (non-
ionized auxin) is the predominant form. When the pH is higher, there is more A– (ionized auxin).
While polar auxin transport distributes the hormone along the longitudinal (up and down) axis of the plant, lateral (side-
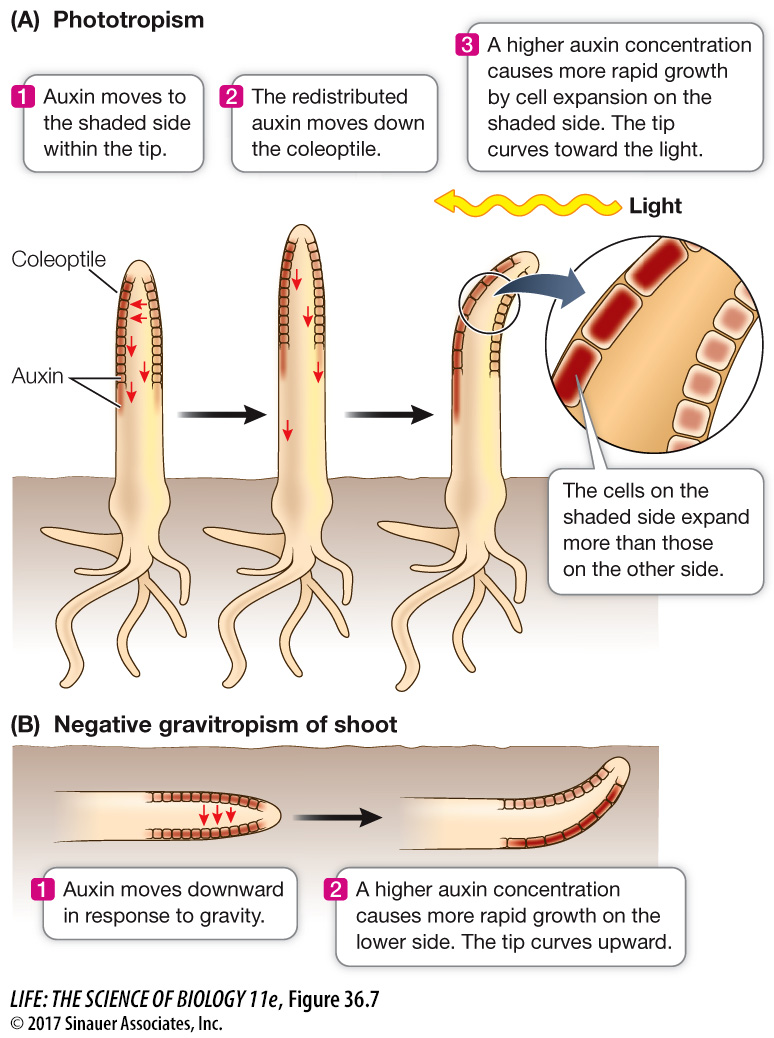
The redistribution of auxin to one side is carried out by auxin carrier proteins that move from the base of the cell to one side; because of this, auxin exits the cell only on that side of the cell, rather than at the base, and moves sideways within the tissue.
This lateral movement of auxin explains the bending of canarygrass seedlings toward light that the Darwins observed. When light strikes a canarygrass coleoptile on one side, auxin at the tip moves laterally toward the shaded side. The asymmetry thus established is maintained as polar transport moves auxin down the coleoptile, so that in the growing region below, the auxin concentration is highest on the shaded side. Cell elongation is thus stimulated on that side, causing the coleoptile to bend toward the light (Figure 36.7A).
774
Light is not the only signal that can cause the redistribution of auxin. Auxin moves to the lower side of a shoot that has been tipped sideways, causing more rapid growth in the lower side and hence an upward bending of the shoot. Such growth in a direction determined by gravity is called gravitropism (Figure 36.7B). The upward gravitropic response of shoots is defined as negative gravitropism; that of roots, which bend downward, is positive gravitropism. Gravitropism in roots also involves differential growth caused by lateral movement of auxin, but the details of the mechanism differ between the root and the shoot.