Apply What You’ve Learned
890
Review
41.1 All white blood cells originate from multipotent stem cells in the bone marrow.
41.5 Two classes of effector T cells (cytotoxic T cells and T-
41.5 Diversity in MHC molecules is very high in humans.
Original Papers: Candotti, F. et al. 2012. Gene therapy for adenosine deaminase-
Gaspar, H. B., A. Alessandro, P. Fulvio, C. Fabio, M. S. Hershfield and L. D. Notarangelo. 2009. How I treat ADA deficiency. Blood 114: 3524−3532.
Hershfield, M. S. et al. 1987. Treatment of adenosine deaminase deficiency with polyethylene glycol-
Severe combined immunodeficiency (SCID) is a very rare inherited condition in which individuals show high susceptibility to viral, fungal, and bacterial infection. Left untreated, it is fatal. SCID can be caused by mutations in any of several genes, one of which codes for the enzyme adenosine deaminase (ADA). ADA is produced in all cells, but the highest levels are found in lymphocytes.
Inside lymphocytes, when DNA is metabolized, toxic deoxyadenosine is produced. When ADA is present, deoxyadenosine is converted to a nontoxic molecule. When ADA is not present, deoxyadenosine accumulates and causes cell death, especially of T cells.
There are currently three treatments for ADA-
| Weeks after initial treatment | Dosage (mL/kg) | Plasma ADA levels (µmol/h/mL) | Percent deoxyadenosine compared with all adenine nucleotides |
|---|---|---|---|
| 0 | 0.1 | 0 | 44 |
| 1 | 0.1 | 2 | 28 |
| 2 | 0.2 | 3 | 19 |
| 3 | 0.2 | 7 | 11 |
| 4 | 0.2 | 8 | 5 |
| 5 | 0.2 | 5 | 4 |
| 6 | 0.2 | 4 | 3 |
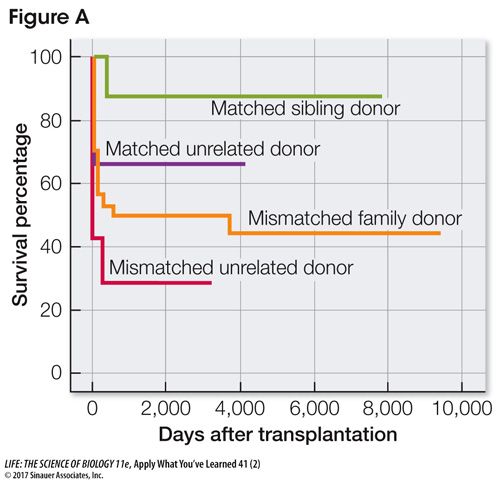
The second treatment method is hematopoietic stem cell transplantation (HSCT), in which stem cells from a donor give rise to lymphocytes that carry the normal ADA gene. These are transplanted into the patient. The greatest obstacle in HSCT is finding a donor whose cell surface markers (in this case, human leukocyte antigens, a type of MHC protein) are similar to those of the patient. To understand the significance of the matching process, 87 patients underwent HSCT with donors having various levels of “matching,” and were studied for long-
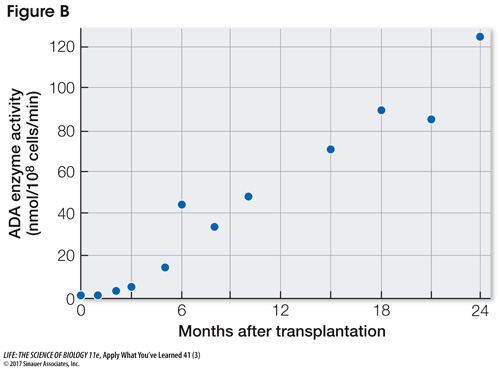
The third treatment for ADA-
Questions
Question 1
According to the table, how many weeks of ERT therapy were needed to restore blood ADA and deoxyadenosine levels to normal? Why were weekly injections necessary? Explain your answer.
By 3 weeks after the initial treatment, ADA and deoxyadenosine levels were normal. Weekly injections were probably needed because ADA gets broken down in blood serum.
Question 2
Summarize the survival of the patients in Figure A from each type of matched donor. Which donor category would most closely approximate what would happen with an untreated patient? Explain your answer.
Matched sibling and matched family donors had almost the same survival percentage: 87% and 88%, respectively. Matched unrelated donors were not nearly as successful, with only 67% survival. However, even this was better than the mismatched family donors, which had only 43% survival. The lowest survival of all was shown by mismatched unrelated donors: 29%. The survivorship of untreated patients might be similar to that with mismatched unrelated donors, but perhaps with even lower survival.
Question 3
Most patients who are successfully treated with gene therapy also receive ERT. Explain how this would be advantageous.
In gene therapy, hematopoietic stem cells are the cells that receive the genetic modification. Thus they are the only cells that will be able to produce and maintain normal levels of ADA. However, all cells produce ADA, because all cells undergo a certain level of DNA metabolism. ERT could keep other body cells from reaching dangerously high levels of deoxyadenosine.
Go to LaunchPad for the eBook, LearningCurve, animations, activities, flashcards, and additional resources and assignments.