Fertilization sets the stage for determination
Unique attributes of amphibian eggs make them ideal models for illustrating how rearrangements of egg cytoplasm set the stage for determination. Remember from Key Concept 19.1 that determination sets the developmental fates of cells, and it precedes differentiation—the expression of different cell properties. The molecules in the cytoplasm of the amphibian egg are not homogeneously distributed. The entry of the sperm into the egg stimulates rearrangements of the egg cytoplasm that introduce additional organization to the egg. Sperm entry establishes the polarity of the zygote, and informational molecules in the egg cytoplasm are organized with respect to that polarity. Therefore, when cell divisions begin, these informational molecules—which guide subsequent development—are not divided equally among daughter cells.
Rearrangement of egg cytoplasm following fertilization is easily observed in some frog species because of pigments in the cytoplasm. The nutrients in an unfertilized frog egg are dense yolk granules that are concentrated by gravity in the lower half of the egg, called the vegetal hemisphere. The haploid nucleus of the egg is located at the opposite end, in the animal hemisphere. The outermost (cortical) cytoplasm of the animal hemisphere is more pigmented than the underlying cytoplasm. The vegetal hemisphere is not pigmented. Because of these differences, it is easy to observe how the cytoplasm rearranges when the egg is fertilized.
The unfertilized frog egg is radially symmetrical. You can turn it on its vegetal–animal pole axis, and all sides are the same. Sperm-binding sites are localized on the surface of the animal hemisphere. When a sperm binds to and enters the egg, the egg’s radial symmetry is converted to bilateral symmetry and a dorsal–ventral axis is created. Cortical cytoplasm rotates toward the site of sperm entry (Figure 43.1). This rotation brings the animal and vegetal regions of cytoplasm into contact with each other, producing a band of lightly pigmented cytoplasm on the side opposite the site of sperm entry. This band, called the gray crescent, marks the location of important developmental events in some species of amphibians.
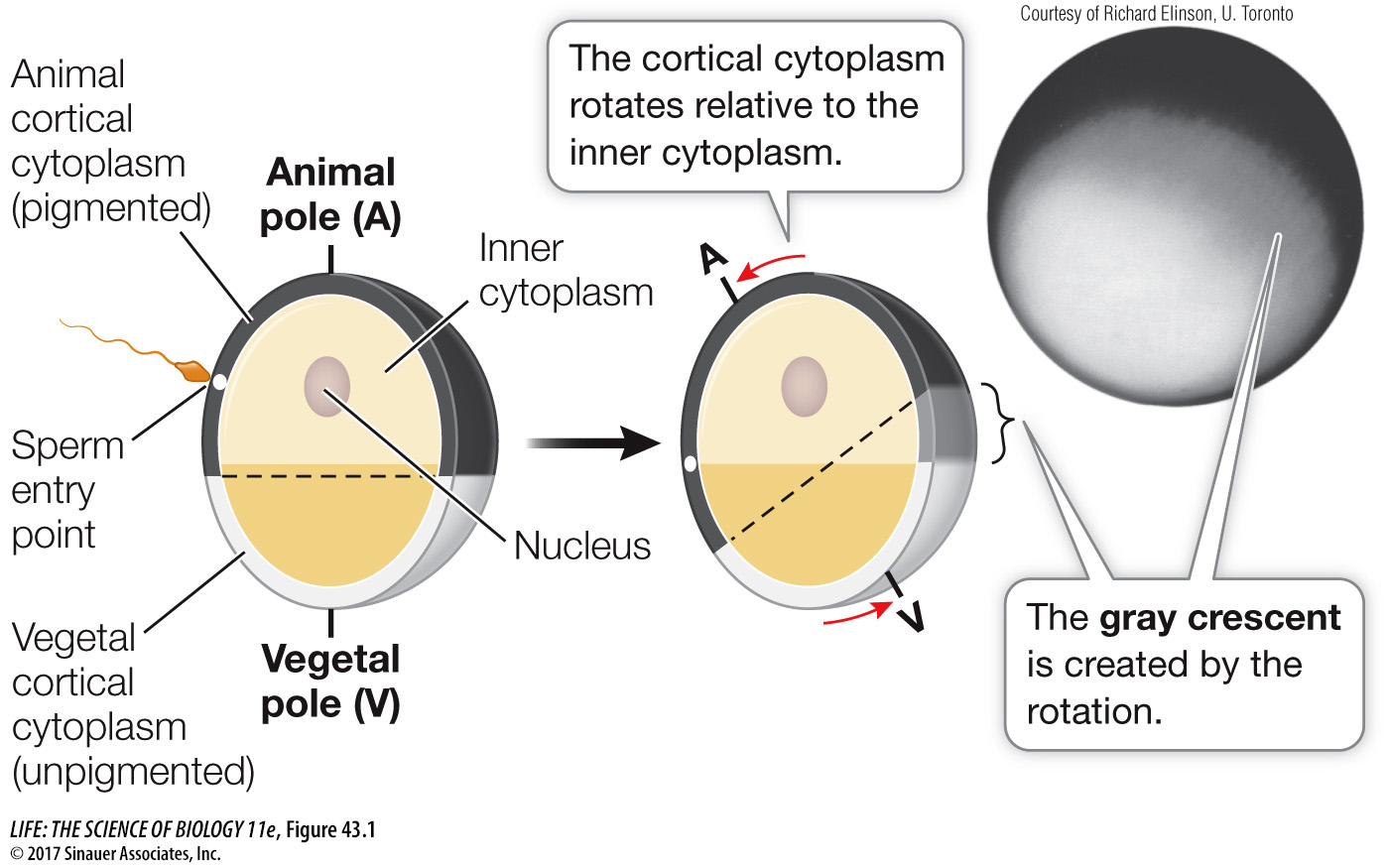
Figure 43.1 The Gray Crescent In amphibian eggs, cortical rotation and rearrangement of the cytoplasm after fertilization create the gray crescent opposite the point of sperm entry. These events are important for specifying the body axes and other important events in later development.
The centriole that was the sperm’s contribution to the egg initiates the cytoplasmic reorganization that coincides with the appearance of the gray crescent. The centriole organizes the microtubules in the vegetal hemisphere cytoplasm into a parallel array that guides the movement of the cortical cytoplasm as well as proteins and organelles.
The movement of cytoplasm, proteins, and organelles changes the distribution of critical signals. A key transcription factor in early development is β-catenin produced from maternal mRNA (mRNA produced and stored in the egg while it was maturing in the ovary). β-Catenin is found throughout the egg cytoplasm (Figure 43.2). Also present throughout the egg cytoplasm is a protein kinase, glycogen synthase kinase-3 (GSK-3), that phosphorylates β-catenin and thereby targets it for degradation. An inhibitor of GSK-3 is localized in the vegetal cortex of the egg. After sperm entry, this inhibitor moves along microtubules to the gray crescent, where it prevents the degradation of β-catenin. As a result, the concentration of β-catenin is higher on the dorsal than on the ventral side of the developing embryo, setting up regional differences within the egg cytoplasm that have developmental consequences.
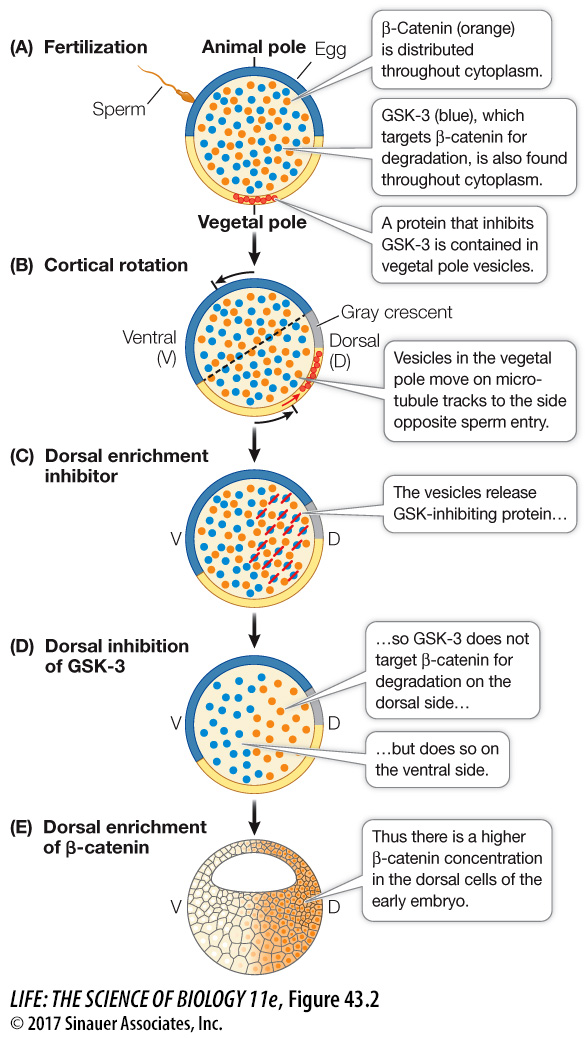
Figure 43.2 Cytoplasmic Factors Set Up Signaling Cascades Cytoplasmic movement changes the distributions of critical developmental signals. In the frog fertilized egg, the interaction of the protein kinase, the GSK-3 inhibitor, and the protein β-catenin results in differential concentrations of β-catenin in blastomeres following cleavage thereby specifying the dorsal–ventral axis of the embryo.

