Cleavage in mammals is unique
Several features of early cell divisions in placental mammals (eutherians) are very different from those seen in other animal groups. First, this process in mammals is very slow. Cell divisions are 12 to 24 hours apart, compared with tens of minutes to a few hours in non-mammalian species. Also, the cell divisions of mammalian blastomeres are not in synchrony with each other. Because the blastomeres do not undergo mitosis at the same time, the number of cells in the embryo does not increase in the regular progression (2, 4, 8, 16, 32, etc.) typical of other species. The slowness of mammalian cleavage means that genes expressed during cleavage can play roles in cleavage. In animals such as sea urchins and frogs where cleavage progresses rapidly, very little if any gene transcription occurs in the blastomeres. Instead, cleavage is directed by molecules that were present in the egg before fertilization.
The pattern of mammalian cleavage is unique and is called rotational cleavage. The first cell division is parallel to the animal–vegetal axis as in radial cleavage, but in the second cell division, the two blastomeres divide at right angles to one other. One blastomere divides parallel to the animal–vegetal axis, while the other divides perpendicular to this axis (Figure 43.4A). As in other animals that have complete cleavage, the early cell divisions in a mammalian zygote produce a loosely associated ball of cells. After the 8-cell stage, however, the behavior of the mammalian blastomeres changes. They change shape to maximize their surface contact with one another, form tight junctions (see Figure 6.7), and become a compact mass of cells (Figure 43.4B).
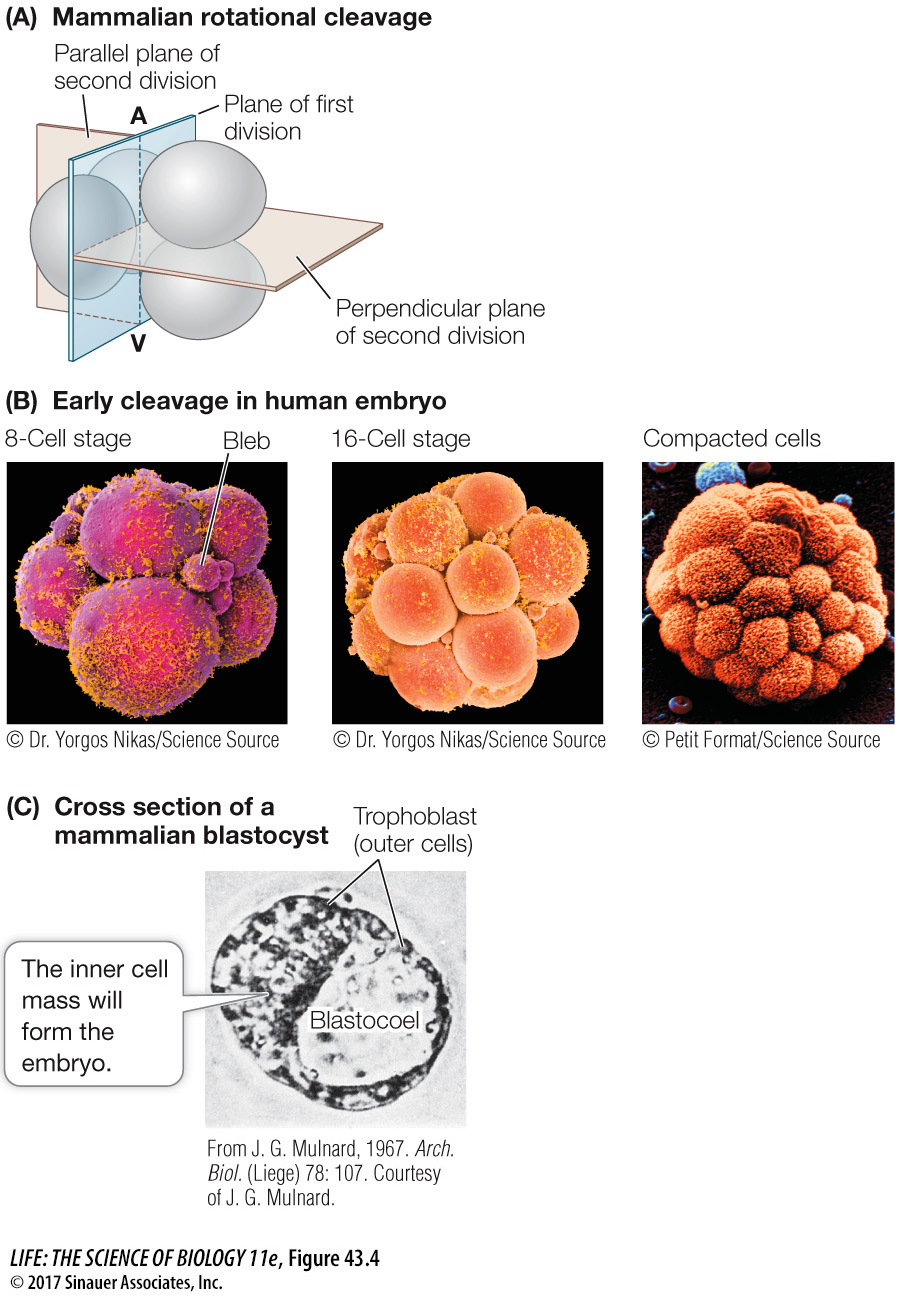
Figure 43.4 Becoming a Blastocyst (A) Mammals have rotational, complete cleavage, in which the plane of the first cleavage is parallel to the animal–vegetal (A–V) axis, but the second cell division involves two planes (beige) at right angles to each other. (B) Scanning electron micrographs (color added) of early cleavage (leading to the formation of the blastocyst) in a human embryo. The cells’ outer surfaces are covered with cilia (bright yellow). The small spheres, or “blebs,” of cytoplasmic material, prominent at the 8-cell stage, disintegrate as cleavage progresses. (C) Seen in cross section under a light microscope, a mammalian blastocyst consists of an inner cell mass adjacent to a fluid-filled blastocoel and surrounded by trophoblast cells.
Soon after the transition to the 32-cell stage, the cells of the mammalian embryo separate into two groups. The inner cell mass will develop as the embryo, while the surrounding outer cells become an encompassing sac called the trophoblast. Trophoblast cells secrete fluid, creating a cavity—the blastocoel—with the inner cell mass at one end. At this stage the mammalian embryo is called a blastocyst, distinguishing it from the blastulas of other animal groups (Figure 43.4C). The pluripotent cells of the inner cell mass are known as embryonic stem cells and are the subject of much research because of their therapeutic potential (see Key Concept 19.1).
Why is mammalian cleavage so different? A key factor is that mammalian eggs contain little or no yolk and must derive all nutrients from the mother. To support the developing embryo, a connection develops between the circulatory systems of the embryo and the mother. As you will see later in this chapter, the structures that provide this connection are the placenta and the umbilical cord. Thus the blastocyst of placental mammals must produce both the embryo (from the inner cell mass) and its support structures (from the trophoblast).
Fertilization in mammals occurs in the upper reaches of the oviduct, and cleavage occurs as the zygote travels down the oviduct to the uterus (Figure 43.5). When the blastocyst arrives in the uterus, the trophoblast adheres to the lining of the uterus (the endometrium), beginning the process of implantation. In humans, implantation begins about 6 days after fertilization and is aided by adhesion molecules and enzymes secreted by the trophoblast.
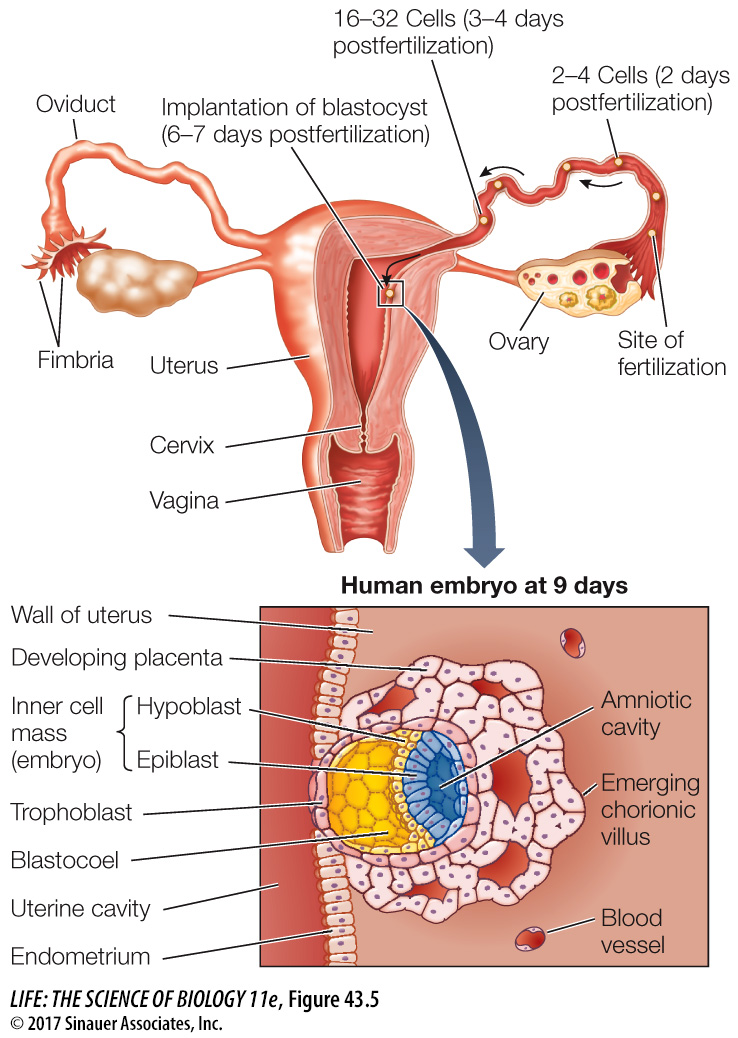
Figure 43.5 A Human Blastocyst at Implantation Adhesion molecules and proteolytic enzymes secreted by trophoblast cells allow the blastocyst to burrow into the endometrium. Once the blastocyst is implanted in the wall of the uterus, the trophoblast cells send out numerous projections—the chorionic villi—which increase the embryo’s area of contact with the mother’s bloodstream. The inner cell mass divides into two embryonic tissues, the hypoblast and the epiblast. The epiblast splits to form the amniotic cavity.
As the blastocyst moves down the oviduct to the uterus, it must not embed itself in the oviduct (Fallopian tube) wall, or the result will be an ectopic, or tubal, pregnancy—a very dangerous condition. Early implantation is prevented by the zona pellucida that surrounded the egg (see Figure 42.6) and remains around the cleaving ball of cells. At about the time the blastocyst reaches the uterus, it hatches from the zona pellucida so implantation can occur.

