The fates of blastomeres depend on the cytoplasm they receive during cleavage
Cleavage results in a repackaging of the egg cytoplasm into a large number of small cells surrounding the fluid-
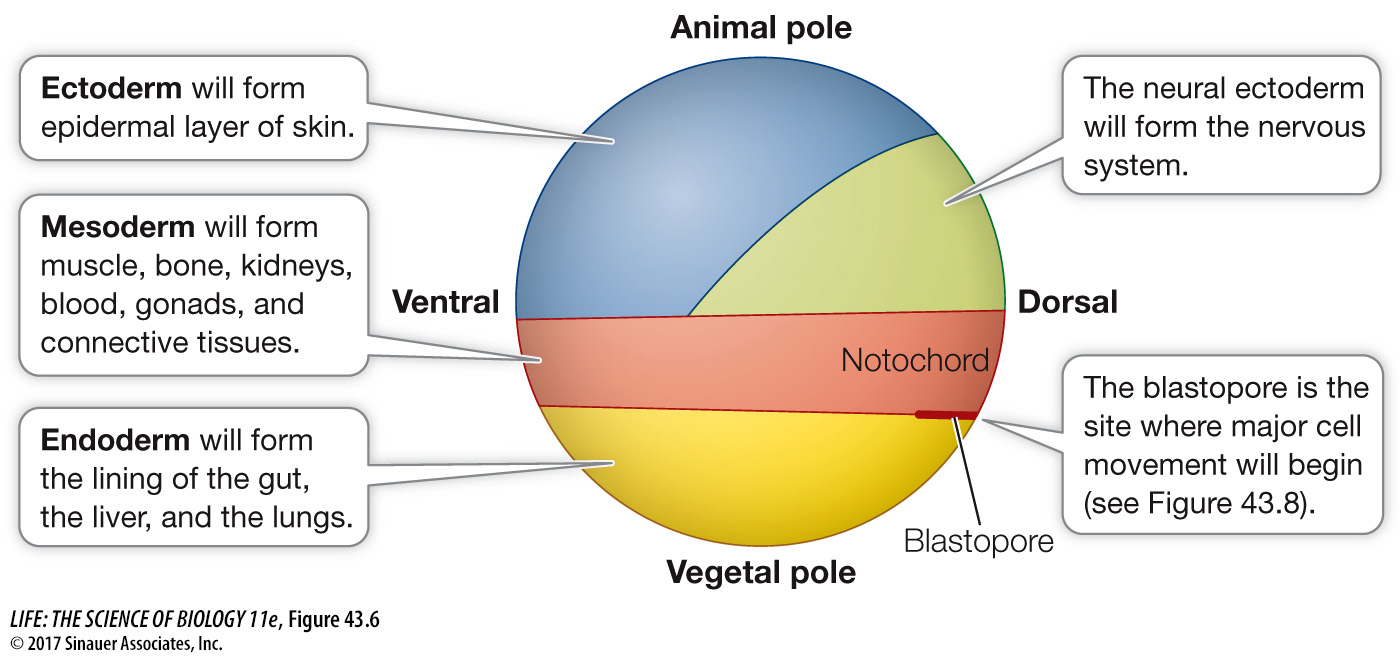
The blastocoel prevents cells from different regions of the blastula from coming into contact and interacting, but that will soon change. During the next stage of development, the cells of the blastula move around and come into new associations with one another, communicate instructions to one another, and begin to differentiate. In many animals, these movements of the blastomeres are so regular and well orchestrated that it is possible to label specific blastomeres with a dye, thus producing fate maps that identify the tissues and organs formed from each blastomere’s progeny (Figure 43.6).
922
Blastomeres become determined—committed to specific fates—
The fates of blastomeres in most vertebrate embryos are determined by information they receive from their environment and their neighboring cells. This mechanism of determination is called regulated specification, and it gives rise to regulative development. Unlike in mosaic development, the loss of some cells during cleavage in regulative development does not affect the developing embryo, because the remaining cells compensate for the loss. A subset of the remaining cells may add a cell division, or they may even change their fate to compensate for the cells lost. Because development is regulative in humans, a single blastomere can be removed from early embryos without harming the remaining blastomeres or disrupting normal development. Cells removed from embryos produced by in vitro fertilization can be used for preimplantation genetic diagnosis to ensure that healthy embryos are selected for implantation in the mother.
If some blastomeres can change their fate to compensate for the loss of other cells during cleavage and blastula formation, can those cells form an entire embryo? Yes, in species with regulative development, they can. If during cleavage or early blastula formation, the blastomeres are physically separated into two groups, both groups can produce complete embryos. Since the two embryos come from the same zygote, they will be monozygotic twins—
Non-