Apply What You’ve Learned
Review
45.3 The ear converts sound waves in air to pressure waves in fluid that bend hair cells to create auditory sensation.
Original Paper: Corcoran, A. J. and W. E. Conner. 2012. Sonar jamming in the field: Effectiveness and behavior of a unique prey defense. Journal of Experimental Biology 215: 4278−4287.
Bats prey on nocturnal moths using echolocation. A bat pursuing prey emits ultrasonic signals that reflect off flying moths and echo back to the bat, enabling it to home in on the prey. Moths can “hear” ultrasonic bat signals through paired auditory organs (tympana) that respond to both low-
Some moths use rapid changes in flight behavior, such as turning away or rapid dives, to evade capture. Others use unique tymbal organs, located in the thorax, to produce ultrasonic clicks. Researchers hypothesized that these clicks serve as echolocation-
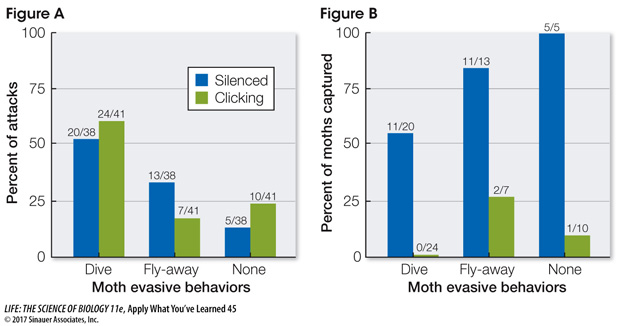
In Figure A, numbers over the bars show the number of attacks when silenced or clicking moths exhibited a particular evasive behavior. Statistically, there was no difference between behaviors or between silenced moths and clicking moths.
In Figure B, numbers over the bars represent the number of captures that were made when silenced or clicking moths exhibited a particular evasive behavior. In all three categories the difference between silenced moths and clicking moths was statistically significant.
Questions
Question 1
According to the data in Figure A, were moths with disrupted tymbal organs still capable of responding to the bats with evasive behaviors? Explain your answer. Why is this important for the validity of this experiment?
According to the data, moths with disrupted tymbal organs displayed the same types of evasive maneuvers as moths with intact tymbal organs. There is no statistical difference in the evasive maneuvers made by silenced versus clicking moths. This finding demonstrates that the moths with the disrupted tymbal organs are still capable of “hearing” the echolocation signals coming from predatory bats and of responding with evasive maneuvers. If a significant difference had been seen in Figure A, this could indicate that tymbal organ disruption impeded a moth’s ability to either hear the bat signals or respond with an evasive behavior.
Question 2
According to the data in Figure B, what effect did disruption of the tymbal organs have on the number of captures? How do these results support the hypothesis that the tymbal organ in B. trigona provides an echolocation-
According to the data, moths with intact tymbal organs were captured significantly less often than moths with disrupted tymbal organs. This is true for all silenced moths no matter what type of evasive maneuver they used to escape bat predation. If evasive maneuvers alone were responsible for moth escapes, we would not expect the number of captures between clicking and silenced moths to be significantly different. The fact that they are significantly different supports the hypothesis that the clicking of the tymbal organ serves as an echolocation-
Question 3
The sound pulses produced by the bats (Myotis sp.) for echolocation in this study have a frequency of around 50 Khz (50,000 cycles per second). The echo from the moth has the same frequency. The upper limit of human hearing is 20,000 Hz. How do you think the basilar membrane in the bat’s cochlea differs in structure from the human basilar membrane?
Different frequency sound waves cause flexion of the basilar membrane at different locations because the basilar membrane varies in thickness and stiffness. At the base it is most stiff and thick and is only flexed by high frequency pressure waves in the cochlear canals. At the apical end it is thinner and less stiff and is flexed by low frequency pressure waves in the cochlear canal. The bat can hear higher frequency sounds than humans can and therefore their basilar membranes must be thicker and stiffer at the basal end than are the basilar membranes of humans.
Go to LaunchPad for the eBook, LearningCurve, animations, activities, flashcards, and additional resources and assignments.