Sliding filaments of actin and myosin cause skeletal muscle to contract
Skeletal muscle is also called striated muscle—
Activity 47.1 The Structure of a Sarcomere
www.life11e.com/
1003
Muscle contraction is due to the interaction between the contractile proteins actin and myosin. Within muscle cells, *actin and myosin molecules are organized into filaments. Actin filaments are also called thin filaments, and myosin filaments are called thick filaments. The two kinds of filaments lie parallel to each other. When muscle contraction is triggered, the actin and myosin filaments slide past each other in a telescoping fashion.
*connect the concepts Key Concept 5.3 discussed how microfilaments made of polymerized actin are elements of the cytoskeleton that are involved in maintaining cell shape and cell movements in all eukaryotic cells.
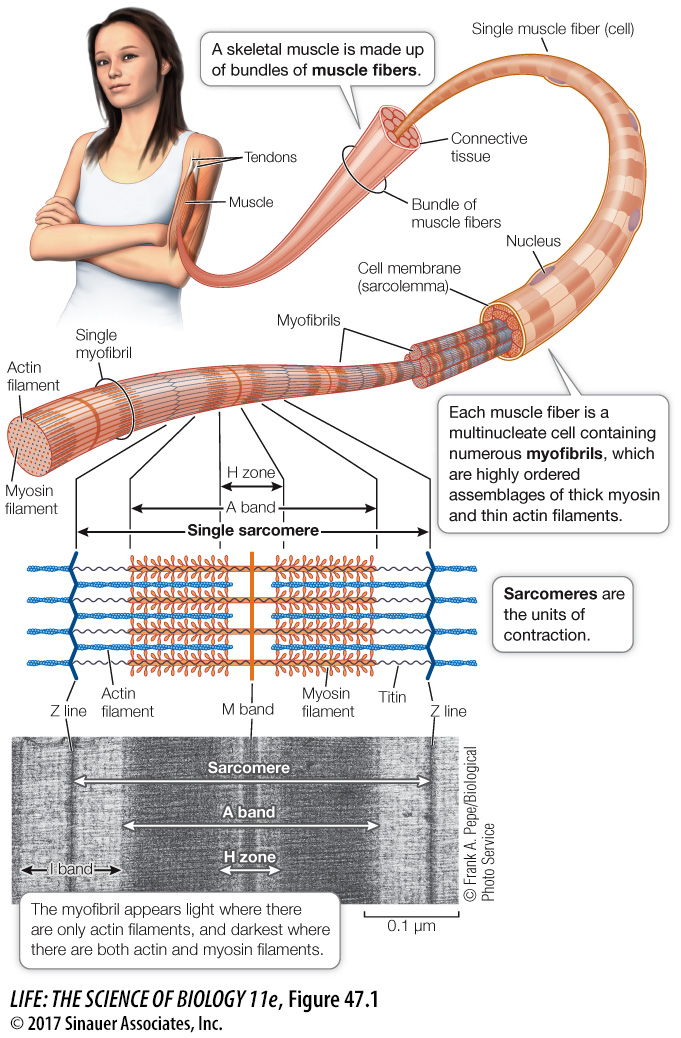
What is the relationship between a skeletal muscle fiber and the actin and myosin filaments responsible for its contraction? Each muscle fiber (cell) is packed with myofibrils—bundles of thin actin and thick myosin filaments arranged in orderly fashion. In most regions of the myofibril, each thick myosin filament is surrounded by six thin actin filaments, and each thin actin filament sits within a triangle of three thick myosin filaments.
A longitudinal view of a myofibril reveals why skeletal muscle appears striated. The myofibril consists of repeating units called sarcomeres. Each sarcomere is made of overlapping filaments of actin and myosin, which create a distinct banding pattern (see Figure 47.1). Before the molecular nature of the muscle banding pattern was known, the bands were given names that are still used today. Each sarcomere is bounded by Z lines, which anchor the thin actin filaments. Centered in the sarcomere is the A band, which contains all the myosin filaments. The H zone and the I band, which appear light, are regions where actin and myosin filaments do not overlap in the relaxed muscle. The dark stripe within the H zone is called the M band; it contains proteins that hold the myosin filaments in their regular arrangement.
The bundles of myosin filaments are held in a centered position within the sarcomere by a protein called titin (see Figure 47.2). Titin is the largest protein in the body; it runs the full length of the sarcomere from Z line to Z line. Each titin molecule runs right through a myosin bundle. Between the ends of the myosin bundles and the Z lines, titin molecules are very stretchable, like bungee cords. In a relaxed skeletal muscle, resistance to stretch is mostly due to the elasticity of the titin molecules.
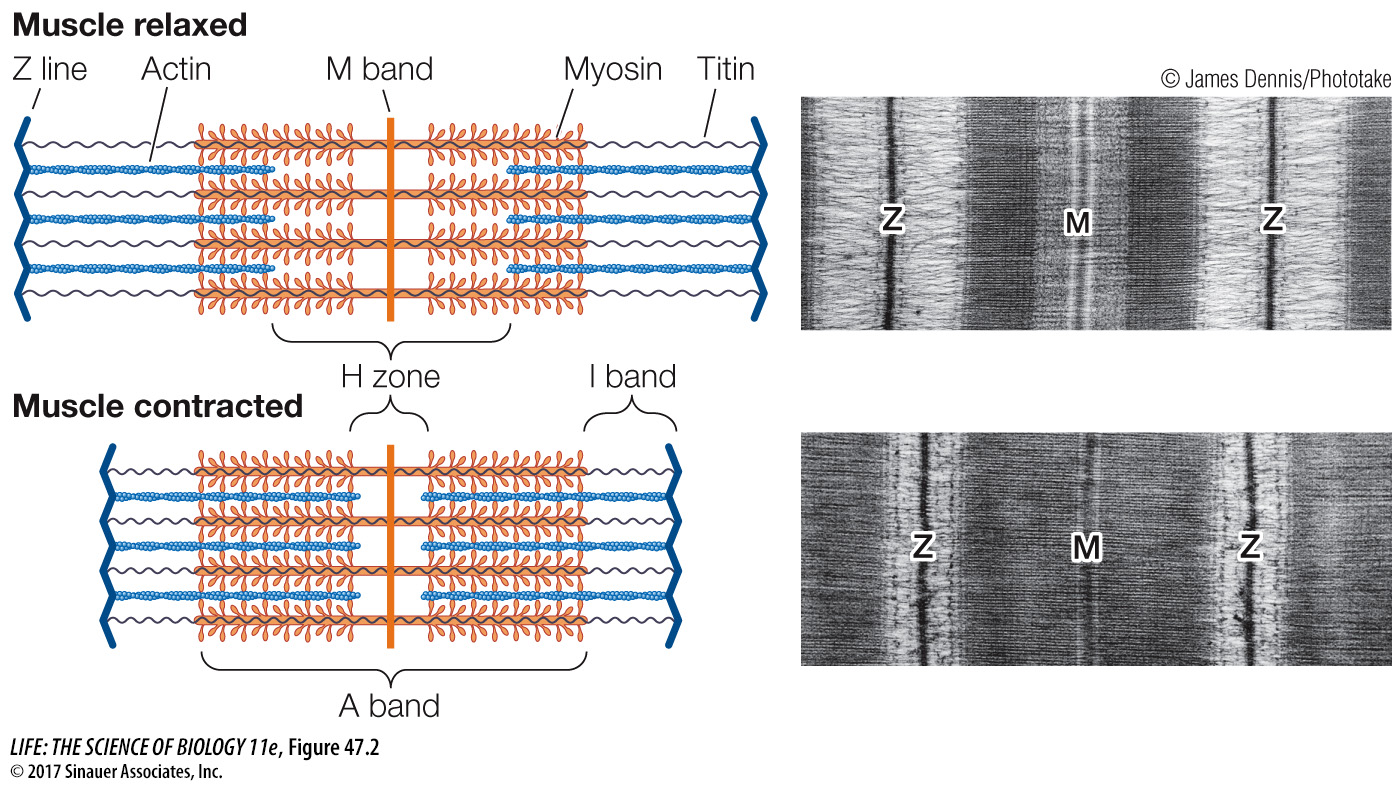
As the muscle contracts, the sarcomeres shorten and the band pattern changes. The H zone and the I band become much narrower, and the Z lines move toward the A band as if the actin filaments were sliding into the H zone, the region occupied by the myosin filaments (Figure 47.2). In the mid-
It is not uncommon in science for critical breakthroughs to be made simultaneously in different laboratories, but in this case the coincidences are remarkable. The leaders of the two teams were named Hugh Huxley and Andrew Huxley—