Cardiac muscle is similar to and different from skeletal muscle
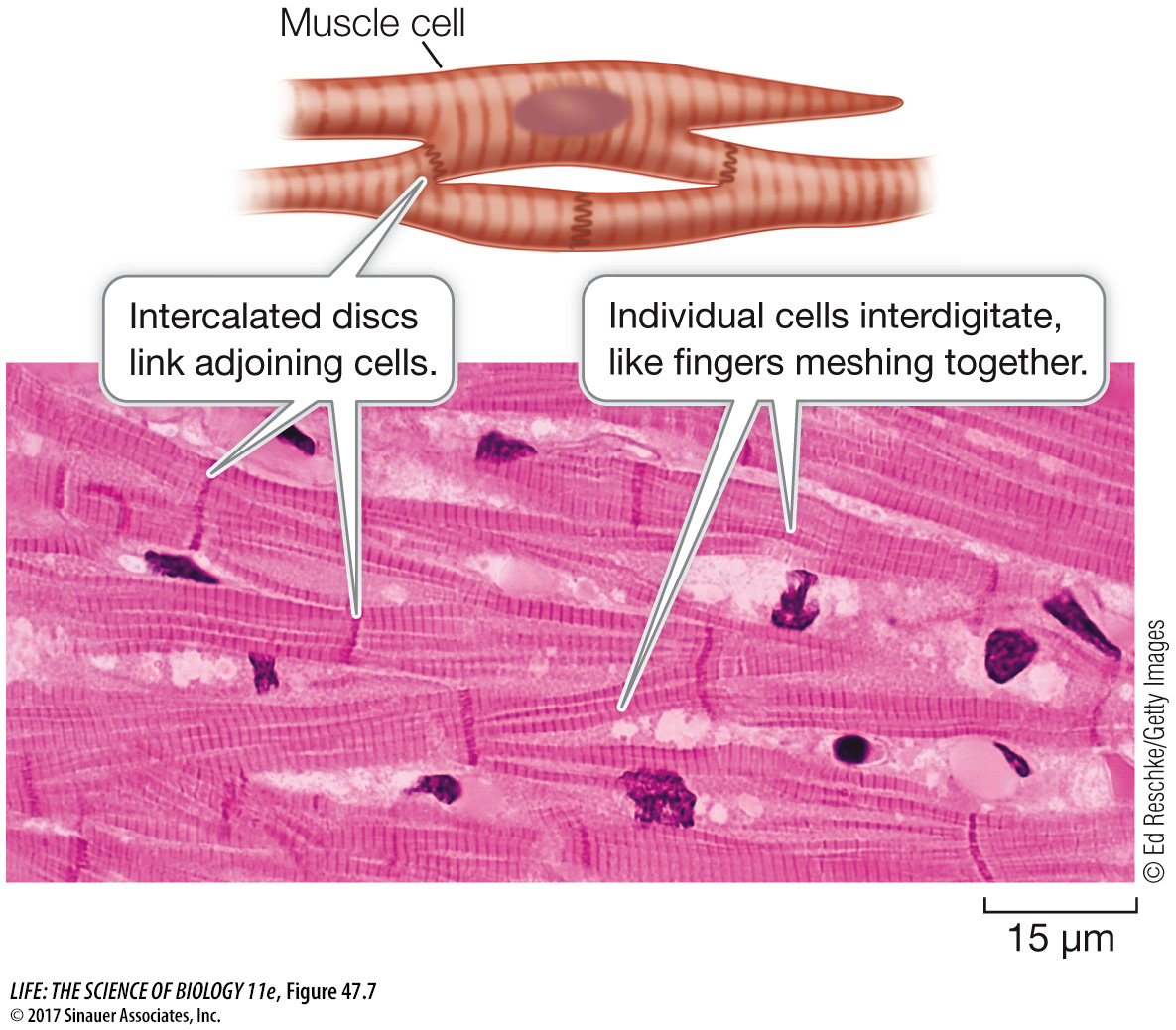
Like skeletal muscle, cardiac muscle appears striated because of the regular arrangement of actin and myosin filaments into sarcomeres (Figure 47.7). The difference between cardiac and skeletal muscle is that cardiac muscle cells are smaller and have only one nucleus each (uninucleate). Cardiac muscle cells branch, and the branches of adjoining cells interdigitate, forming a meshwork resistant to tearing. As a result, the heart walls can withstand high pressures while pumping blood, without the danger of developing leaks. Adding to the strength of cardiac muscle are intercalated discs that provide strong mechanical adhesions between adjacent cells. Gap junctions are an important feature of cardiac muscle. These structures in the intercalated discs allow cytoplasmic continuity between cells (see Figure 7.16). Because of gap junctions, cardiac muscle cells are electrically coupled. An action potential initiated at one point in a sheet of cardiac muscle spreads rapidly, causing a large number of cardiac muscle cells to contract simultaneously.
1006
Certain cardiac muscle cells are specialized for generating and conducting electric signals. These pacemaker and conducting cells have a low density of actin and myosin filaments, but they initiate and coordinate the rhythmic contractions of the heart. (The molecular basis for this pacemaking function will be covered in Key Concept 49.3.) Pacemaker cells make the vertebrate heartbeat autorhythmic, meaning it is generated by the heart muscle itself. A heart removed from a vertebrate can continue to beat with no input from the nervous system. Input from the autonomic nervous system modifies the rate of the pacemaker cells, but it is not essential for their continued rhythmic function.
1007
The mechanism of excitation–
Media Clip 47.1 Be Still My Beating Stem Cell Heart
www.life11e.com/