Regulating breathing requires feedback
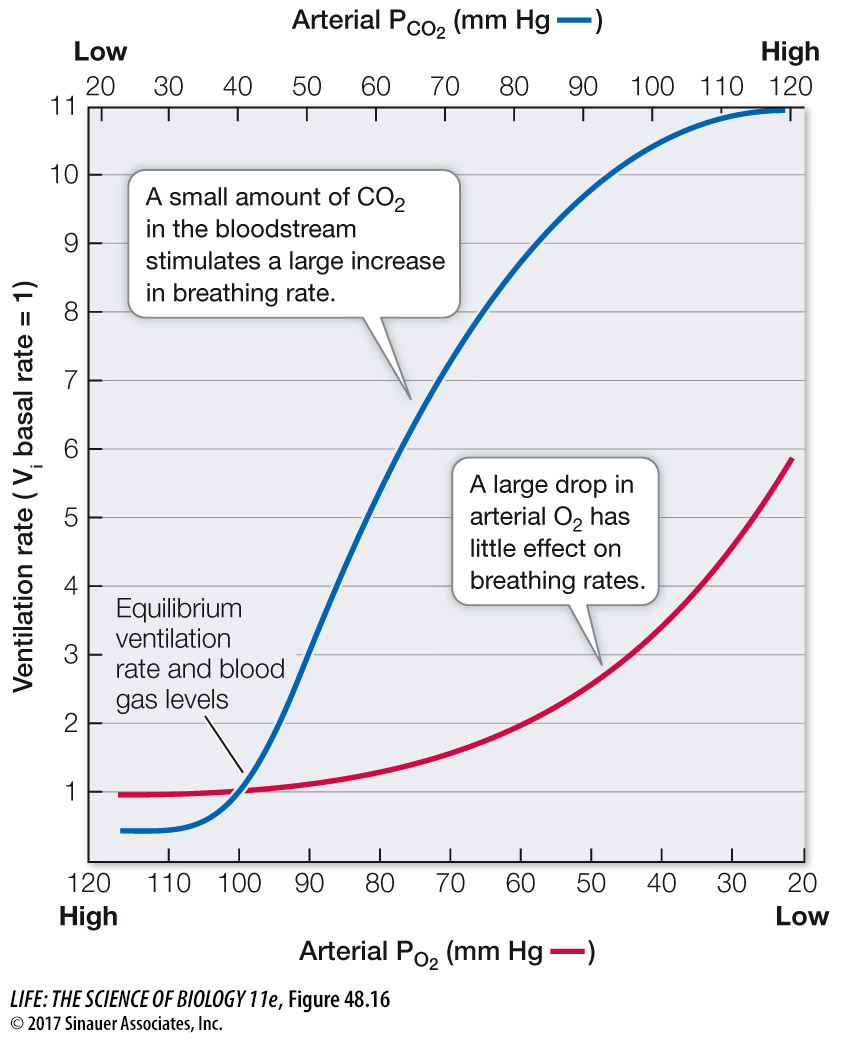
When breathing or metabolism changes, the PO2 and PCO2 in the blood change as well. It is reasonable to surmise that the blood levels of one or both of these gases provide feedback information to the breathing rhythm generator in the medulla. Humans and other mammals are remarkably insensitive to falling levels of O2 in arterial blood but are extremely sensitive to increases in CO2. That is, arterial PO2 can deviate considerably from normal without causing much of an increase in ventilation rate, but even a small rise in arterial PCO2 causes a large increase in ventilation (Figure 48.16). This relationship is reversed for water-
1040
The lack of O2 sensitivity in humans was tragically demonstrated by an accident at the Kennedy Space Center in 1981 prior to the first flight of the Space Shuttle Program. The space shuttle Columbia was being prepared for launch, and to reduce the danger of fire, the engine compartment was purged with nitrogen and sealed. Unaware of the nitrogen, workers opened a hatch to the compartment for inspection. The first worker to enter became unconscious, and others followed him in a rescue attempt. Three of these workers died, and two others who were stricken recovered. When exposed to lack of oxygen, there is no alerting, emergency response—
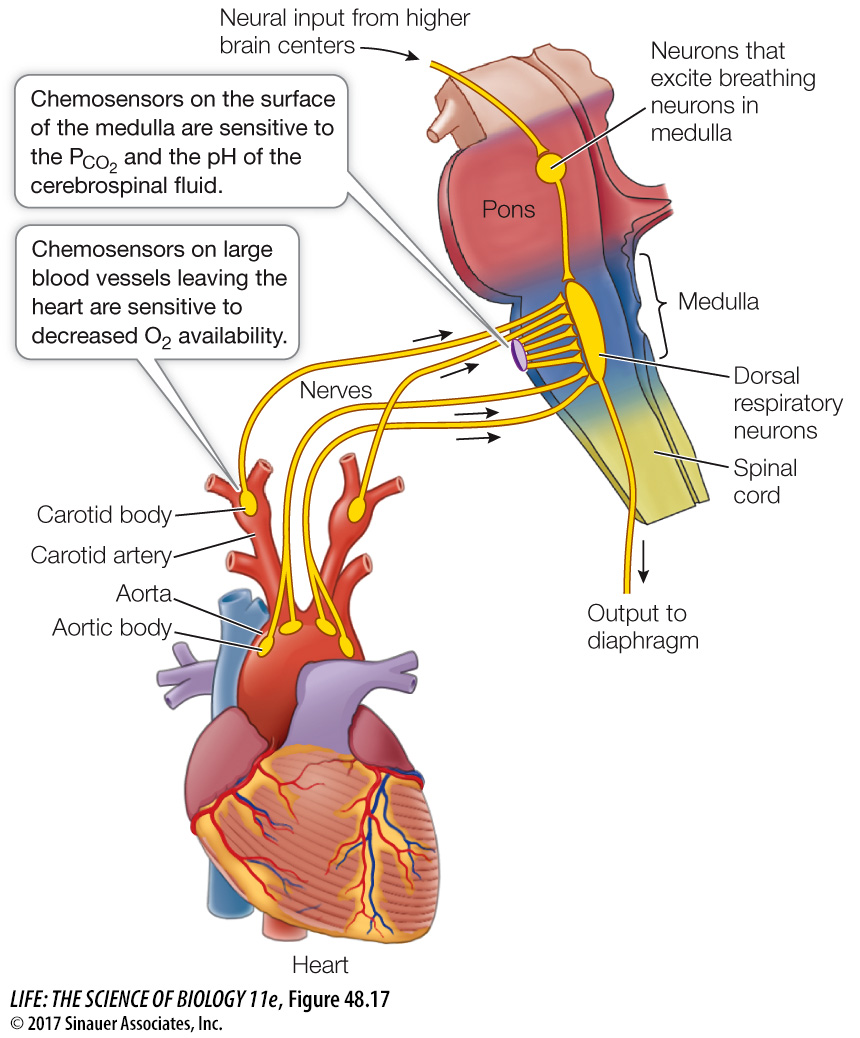
Where are partial pressures of gases in the blood sensed? The major site of PCO2 sensitivity is an area on the ventral surface of the medulla. The primary sensitivity of these chemosensitive cells is not, however, to CO2. It is H+ ions that stimulate these cells. The H+ ion concentration in the environment of these cells is a direct reflection of the PCO2 of the blood. When the PCO2 of the blood is higher than that of the extracellular fluid in this area, CO2 diffuses out of the blood. This CO2 interacts with H2O in the plasma to form carbonic acid (H2CO3), which dissociates into H+ ions and HCO3– ions (see Figure 48.14, which describes this same reaction in the RBCs). It is the H+ ions produced by the dissociation of carbonic acid that stimulate the chemosensitive cells that increase respiratory gas exchange. Thus even though we measure blood PCO2 as the stimulus for breathing, the major stimulus is an increase in H+ ion concentration (lowered pH).
We do have some physiological sensitivity to blood PO2 in nodes of neural tissue on the large blood vessels leaving the heart: the aorta and the carotid arteries (Figure 48.17). These carotid bodies and aortic bodies are chemosensors. If the blood supply to these structures decreases, or if the blood PO2 falls dramatically, the chemosensors are activated and send nerve impulses to the breathing control center. Although we are not very sensitive to changes in blood PO2, the carotid and aortic bodies can stimulate increases in breathing during exposure to high altitudes or when blood volume or blood pressure is very low. Also, there is a synergism between CO2 and O2 sensing. When blood PCO2 increases, there is an increased sensitivity to low O2, and vice versa.