Fick’s law applies to all systems of gas exchange
Whether in air or water, the diffusion rates of respiratory gases depend on their partial pressure gradients and on other factors that can be described quantitatively with a simple equation called Fick’s law of diffusion. All environmental variables that limit respiratory gas exchange and all adaptations that maximize respiratory gas exchange are reflected in one or more components of this equation. Fick’s law is written as
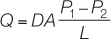
where
Q is the rate at which a gas such as O2 diffuses between two locations.
D is the diffusion coefficient, which is a characteristic of the diffusing substance, the medium, and the temperature. For example, perfume has a higher D than motor oil vapor, and all substances diffuse faster at higher temperatures and faster in air than in water. Temperature is not expressed explicitly in Fick’s law because the diffusion coefficient is usually determined at room temperature (about 20°C).
1024
A is the area across which the gas is diffusing.
P1 and P2 are the partial pressures of the gas at the two locations.
L is the path length, or distance, between the two locations.
(P1 – P2)/L is a partial pressure gradient.
The strict dependence of animals on diffusion for gas exchange with their environments has selected for various adaptations that maximize Q, many of which we will describe in this chapter. Animals can maximize D for respiratory gases by using air rather than water as their gas exchange medium whenever possible. All other adaptations for maximizing respiratory gas exchange must influence the surface area (A) for gas exchange or the partial pressure gradient across that surface area.