The heartbeat originates in the cardiac muscle
1052
Cardiac muscle has unique adaptations that enable it to function as a pump. Cardiac muscle cells are in electrical contact with one another through gap junctions that enable action potentials to spread rapidly from cell to cell. Because a spreading action potential stimulates contraction, large groups of cardiac muscle cells contract in unison. This coordinated contraction is essential for pumping blood effectively.
Some cardiac muscle cells are pacemaker cells that initiate action potentials without stimulation from the nervous system. When they fire action potentials, they stimulate neighboring cells to contract. The primary pacemaker of the heart is a group of modified cardiac muscle cells, the sinoatrial node, located at the junction of the superior vena cava and right atrium (see Figure 49.8). The resting membrane potentials of these cells are less negative than those of other cardiac muscle cells and are not stable; instead they gradually become even less negative until they reach threshold for initiating an action potential. The action potentials of pacemaker cells are very different from those of neurons and other muscle cells (see Figure 44.8). They are slower to rise; they are broader; and they are slower to return to resting potential (Figure 49.6A). These properties of pacemaker cells are due to the ion channels in their membranes.
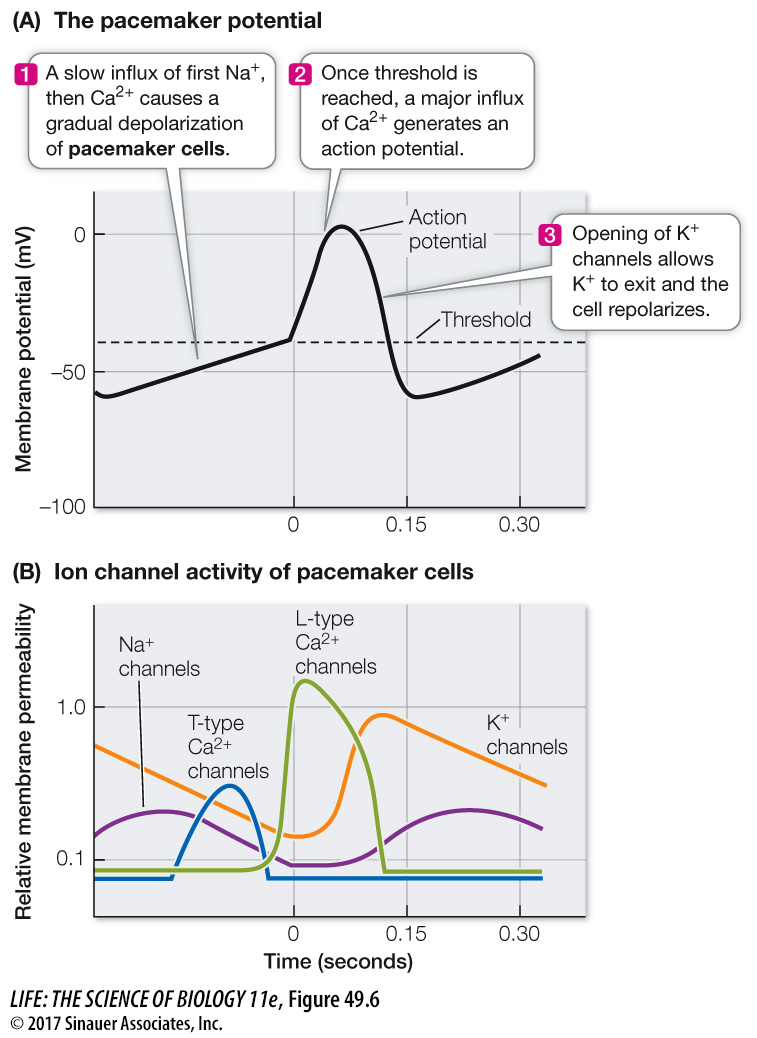
Pacemaker potentials involve Na+, Ca2+, and K+ channels (Figure 49.6B). As discussed in Key Concept 44.2, when Na+ or Ca2+ channels open, positive charges flow into the cell and the membrane potential becomes less negative. When K+ channels open, positive charges flow out of the cell and the membrane potential becomes more negative. Because the Na+ channels of pacemaker cells are open more of the time than are those of other cardiac muscle cells, the pacemaker resting potential is less negative. The action potential of pacemaker cells is due to voltage-
The unstable resting potential of pacemaker cells is due to the behavior of cation channels. As in neurons and skeletal muscle cells, the rise of the action potential is followed by opening of voltage-
1053
The gradual rise in membrane potential closes the channels that allow Na+ to move into the cell, but as the membrane becomes less negative, some Ca2+ channels open, causing the membrane potential to continue its gradual rise. These Ca2+ channels are called T-
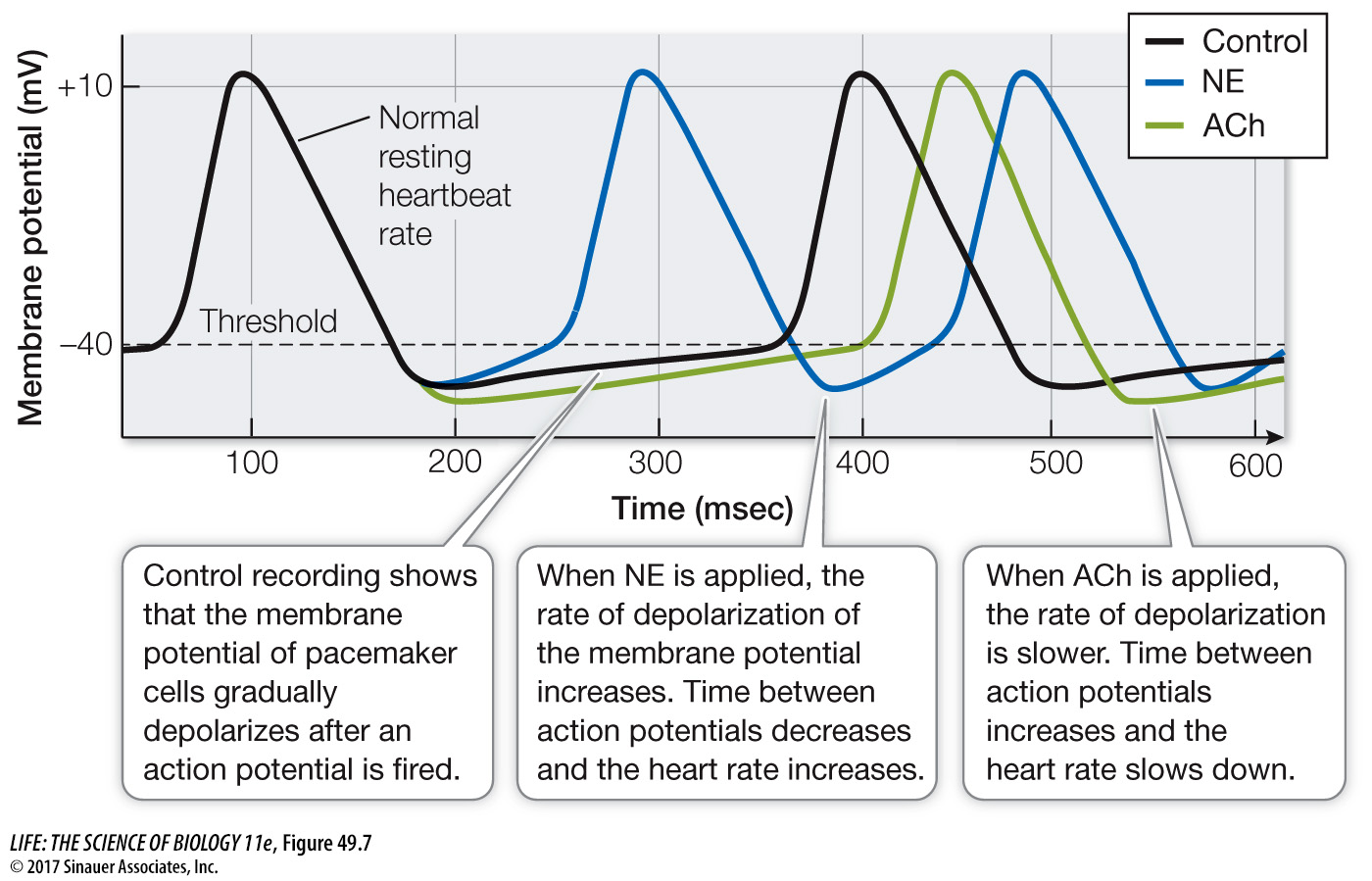
The autonomic nervous system controls the heartbeat (speeds it up or slows it down) by influencing the rate at which the membrane potentials of pacemaker cells drift upward (Figure 49.7). Norepinephrine (NE) released onto pacemaker cells by sympathetic nerves increases the permeability of the Na+ channels and the Ca2+ channels. The result is that the resting potential of the pacemaker cells drifts up more rapidly, the interval between action potentials is decreased, and the heart beats faster. Conversely, the parasympathetic neurotransmitter acetylcholine (ACh) has opposite effects. ACh increases the permeability of K+ channels so that the membrane potential becomes even more negative following an action potential and rises more slowly. ACh also decreases the permeability of the Ca2+ channels so that the rate of rise of the membrane potential slows, the interval between pacemaker action potentials lengthens, and the heart slows down.