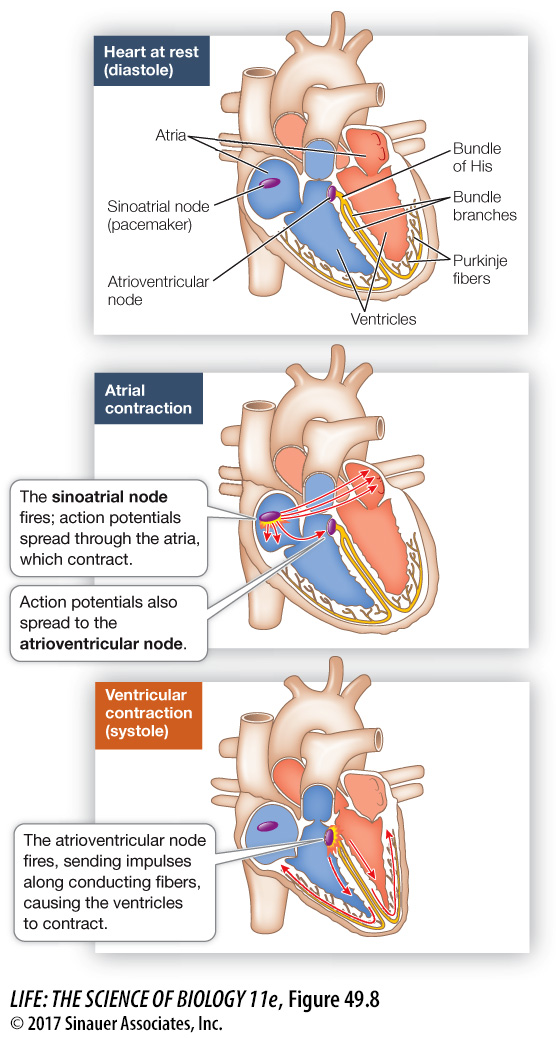
A conduction system coordinates the contraction of heart muscle
A normal heartbeat begins with an action potential in the sinoatrial node (Figure 49.8). This action potential spreads rapidly throughout the electrically coupled cells of the atria, causing them to contract in unison. Because there are no gap junctions between the cells of the atria and those of the ventricles, the action potential does not spread directly to the ventricles. Therefore the ventricles do not contract in unison with the atria.
1054
investigating life
Silencing Mutant Myosin Genes
experiment
Original Paper: Jiang, J., H. Wakimoto, J. B. Seidman and C. E. Seidman. 2013. Allele-
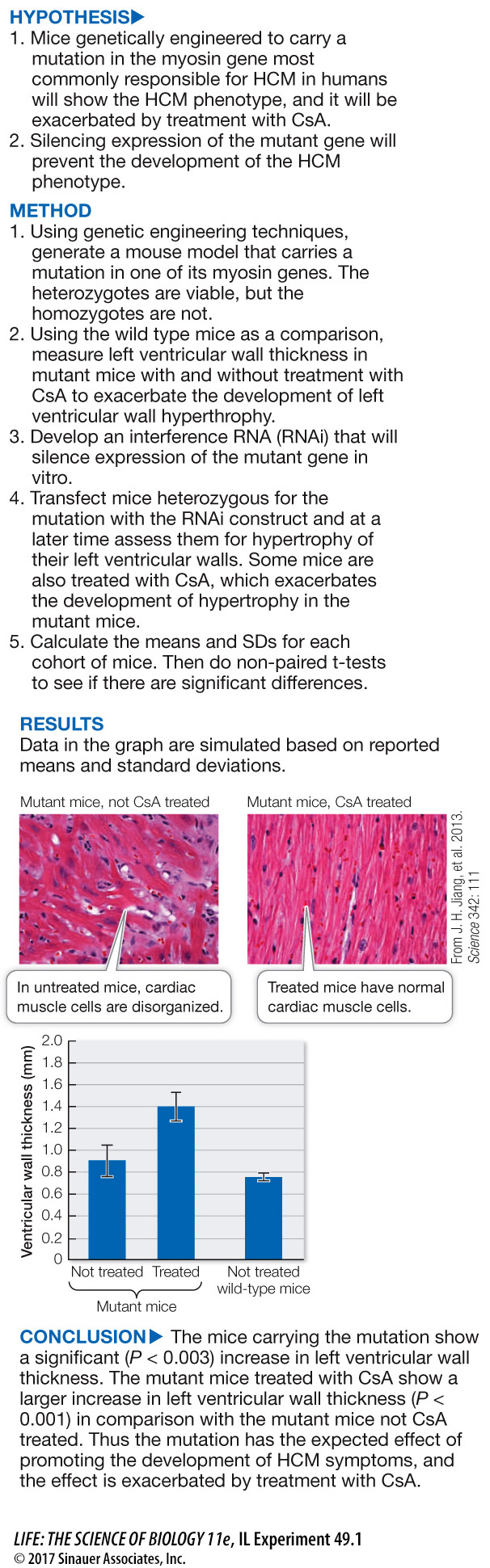
Hypertrophic cardiomyopathy (HCM) is caused by mutations in myosin genes. A similar mutation has been engineered into mice, and they develop HCM. In addition, it was shown that treatment of these mice with cyclosporine A (CsA) exacerbates their development of HCM. Since the HCM model mice are heterozygous for the mutated gene (as are most afflicted humans), and since there are multiple myosin genes, the question arose as to whether or not the selective silencing of the mutant gene would allow the normal alleles to correct the disorganization of the cardiac muscle due to the mutant allele.
work with the data
The second hypothesis posed in the experiment above is that treatment of the mutant mice with interference RNA (RNAi) targeted to the mutant myosin gene will reduce the development of the HCM phenotype. The data are listed in the table. All values are for left ventricular wall thickness (mm) of individual mice and are simulated based on the reported means and SDs.
| CsA treated | Not CsA treated | RNAi and CsA treated | RNAi but not CsA treated |
|---|---|---|---|
| 1.60 | 0.96 | 0.87 | 0.63 |
| 1.34 | 0.98 | 0.82 | 0.68 |
| 1.50 | 1.01 | 0.87 | 0.85 |
| 1.39 | 0.89 | 0.91 | 0.65 |
| 1.50 | 0.86 | 0.74 | 0.68 |
| 1.44 | 0.91 | 0.83 | |
| 1.42 | 0.92 |
QUESTIONS
Question 1
Comparing mutant mice that did not receive CsA, did the treatment with RNAi significantly reduce their left ventricular wall thickness?
Comparing mutant mice not CsA treated, but treated with or without RNAi, the left ventricular wall thickness (LVWT) of the controls was 0.96 ± 0.12 and that of the RNAi-
Question 2
Comparing mutant mice that did receive CsA, did the treatment with RNAi significantly reduce their left ventricular wall thickness?
Comparing mutant mice that did receive CsA but did or did not receive RNAi treatment, the LVWT of the control mice was 1.66 ± 0.22 and that of the RNAi mice was 0.85 ± .06. A t-test calculates P < 0.001, indicating that these two mean LVWTs are significantly different.
A similar work with the data exercise may be assigned in LaunchPad.
How does the action potential move from the atria to the ventricles? Situated at the junction of the atria and the ventricles is a nodule of modified cardiac muscle cells—