Insulin and glucagon control fuel metabolism
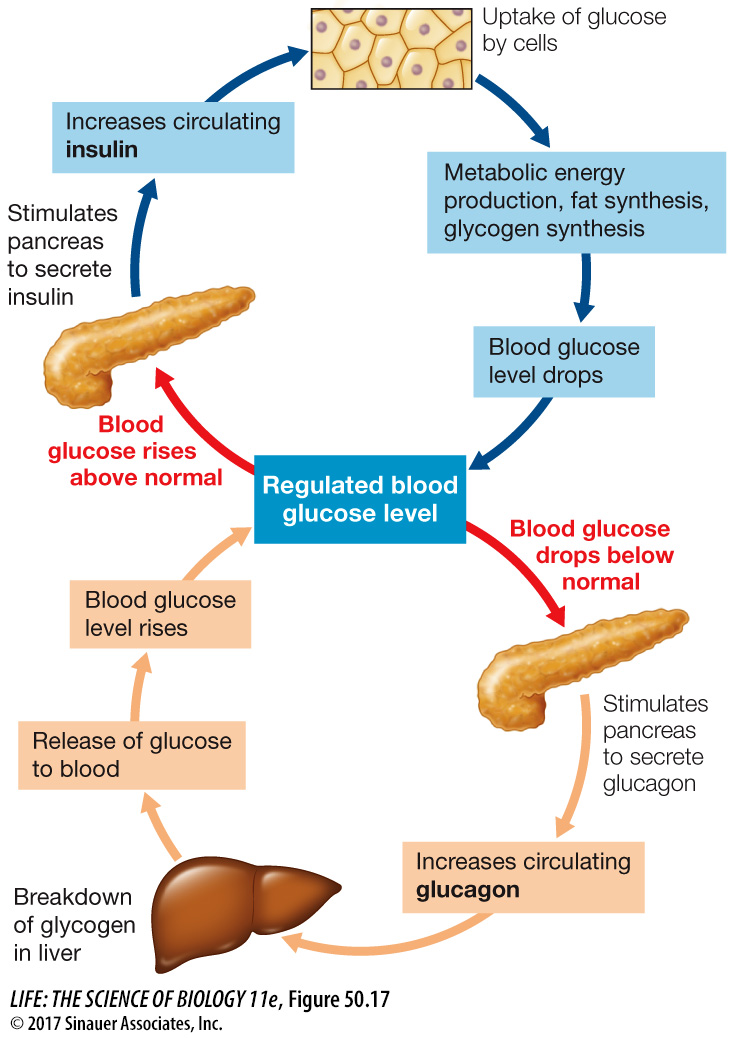
During the absorptive state, blood glucose levels rise as carbohydrates are digested and absorbed (Focus: Key Figure 50.17). During this time, the pancreas releases the hormone insulin which plays a major role in directing glucose to where it will be used or stored. In Key Concept 50.3 we mentioned that the pancreas has both an exocrine function (secretions involved in digestion) and endocrine functions. The endocrine functions are located in clusters of cells in the pancreas called the islets of Langerhans. One class of islet cells, the beta cells, produces and releases insulin. Another class, the alpha cells, produces and releases the hormone glucagon (discussed below). The actions of insulin vary in different tissues, but they are all aimed at promoting the use of glucose for metabolic fuel and directing the excess glucose into storage as either glycogen or fat.
focus: key figure
Question
Q:The nervous system depends on a constant supply of glucose, yet none of the actions of insulin or glucagon in this figure refer to supplying glucose to the nervous system. Why?
The uptake of glucose by cells of the nervous system does not depend on hormonal stimulation—
Animation 50.2 Insulin and Glucose Regulation
www.life11e.com/
1088
Glucose enters cells by diffusion. This diffusion is facilitated by transporters, but they are not active transporters—
Insulin plays many roles in controlling how cells use the glucose they take up from the circulation. In adipose cells, insulin inhibits lipase and promotes fat synthesis from glucose. In the liver, insulin activates an enzyme, glucokinase, that phosphorylates glucose as it enters the liver cell so it cannot diffuse back out again, thereby enhancing the overall diffusion of glucose into the cells. At the same time, insulin inhibits the enzyme glucose phosphatase that enables glucose to leave the cell. Insulin also activates glycogen synthase, an enzyme in liver cells that catalyzes the incorporation of glucose into glycogen, and activates enzymes that increase the flow of glucose into glycolysis (Figure 50.18).
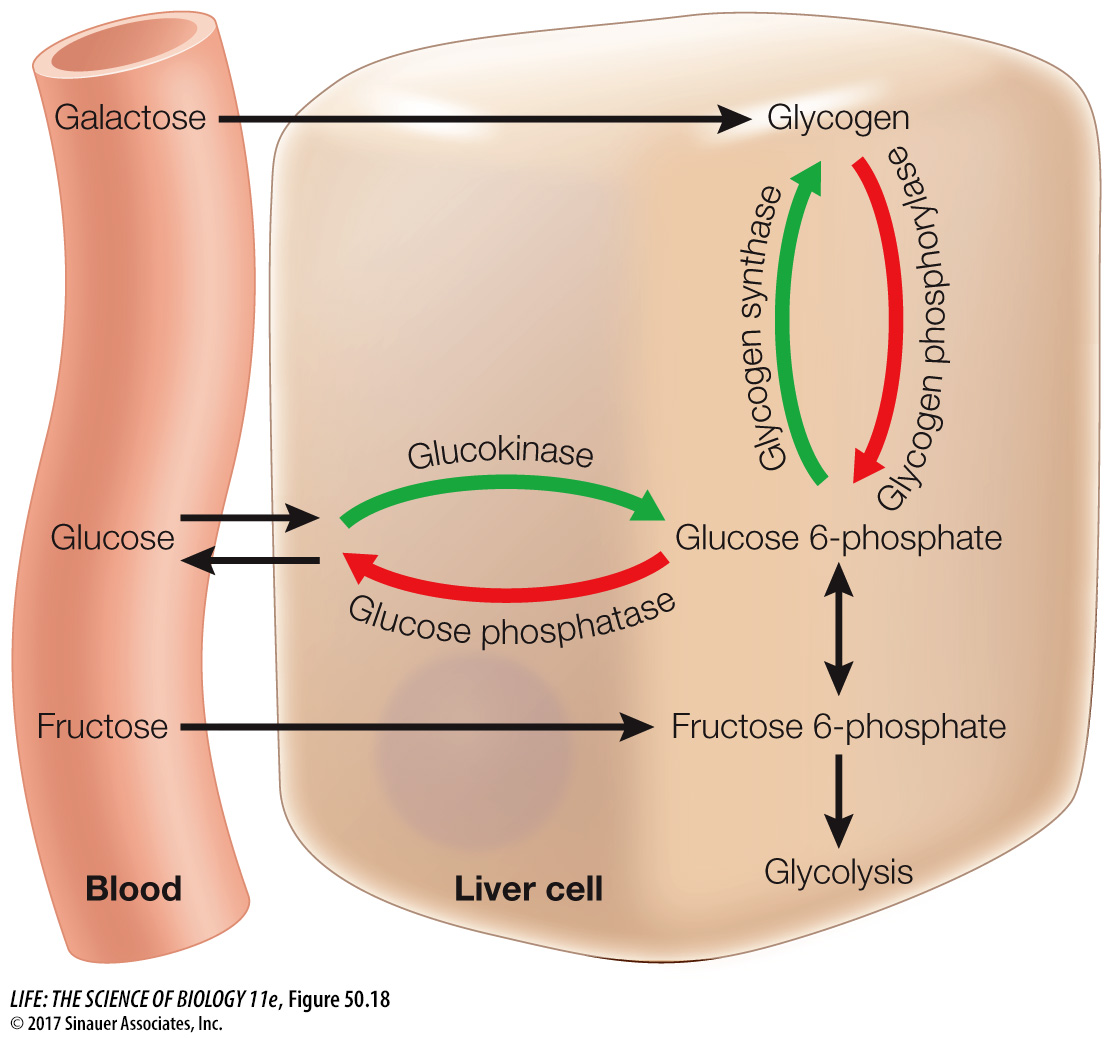
To maintain blood glucose levels during the postabsorptive state, liver cells break down their stored glycogen, releasing glucose into the blood. The multiple processes that make it possible for the liver to release glucose depend on a fall in blood insulin levels (see Figure 50.18). Falling insulin inhibits the enzyme responsible for glycogen synthesis and activates the enzyme that breaks down glycogen. Also, with less insulin, the enzyme that phosphorylates glucose is inhibited and activity of glucose phosphatase is increased. The result is a breakdown of glycogen and the return of glucose to the blood. Another consequence of the fall in insulin levels is the increased activity of lipases in the liver and adipose tissue, releasing fatty acids to the blood. Most cells preferentially use fatty acids as their metabolic fuel during the postabsorptive state. Overall, the most important control of fuel metabolism in the postabsorptive state is the lack of insulin.
One tissue that does not switch fuel sources when an animal is postabsorptive is the nervous system. The cells of the nervous system require a constant supply of glucose and can use other fuels only to a very limited extent. Most neurons do not require insulin to absorb glucose from the blood, but they do need an adequate glucose concentration gradient to drive the facilitated diffusion of glucose across their cell membranes. Therefore it is critical that blood glucose levels are maintained when an animal is postabsorptive. The overall dependence of neural tissues on glucose, and their requirement for constant blood glucose levels, are the reasons it is so important for other cells of the body to shift to fat metabolism during the postabsorptive state.
The metabolism of fuel molecules during the postabsorptive state is mostly controlled by the lack of insulin, but if blood glucose falls below a certain level, glucagon is released. Glucagon’s effect is opposite that of insulin: it stimulates liver cells to break down glycogen and to carry out gluconeogenesis. Thus, under the influence of glucagon, the liver produces glucose and releases it into the blood. Note that under conditions that stimulate glucagon release, the effects of low insulin are already in play—