The kidneys help regulate acid–
Besides regulating salt and water balance and excreting nitrogenous wastes, the kidneys have another important role: they regulate the hydrogen ion concentration (the pH) of the extracellular fluids. pH is a critical variable because it influences the structure and function of proteins.
One way to minimize pH changes in a chemical solution is to add a buffer—
CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3–
From this equation, you can see that if excess hydrogen ions are added to this reaction mixture, the reaction will move to the left and absorb the excess H+. If hydrogen ions are removed from the reaction mixture, however, the reaction will move to the right and supply more H+.
The HCO3– buffer system is important for controlling the pH of the blood, and therefore of the interstitial fluids as well, because the reaction can be pushed to the right and pulled to the left physiologically. The lungs control the levels of CO2 in the blood, thus altering the acid portion of the reaction. CO2 is considered the acid portion of the reaction because if you add additional CO2, the reaction shifts to the right, producing more H+ ions. The kidneys control the base portion of the reaction by removing H+ from the blood and returning HCO3– to the blood. How does this occur?
HCO3– is filtered in the glomerulus and is therefore present in the tubule fluid. As illustrated in Figure 51.11A, tubule cells transport H+ into the tubule fluid in exchange for Na+. In the tubule, the excreted H+ combines with the filtered HCO3– to produce H2CO3 that then disassociates into H2O and CO2. The CO2 diffuses into the tubule cells, where in the presence of the enzyme carbonic anhydrase it produces HCO3– that is transported out of the basal end of the cell into the interstitial fluid and thence to the blood. Thus for each H+ secreted into the tubule fluid, a HCO3– ion is released into the blood.
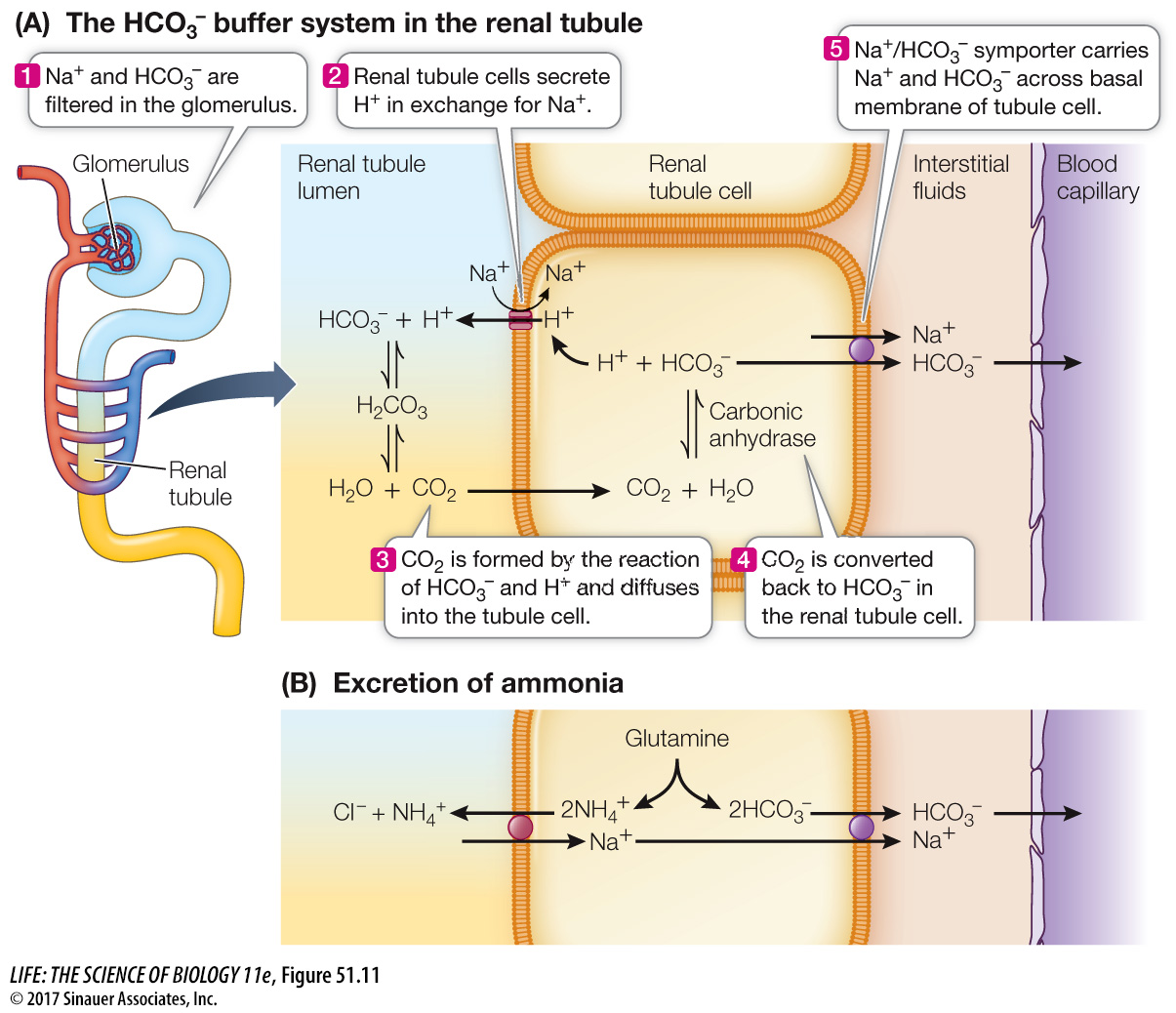
Another mechanism for H+ secretion and HCO3– reabsorption involves ammonium ions (NH4+). The metabolism of glutamine in tubule cells produces NH4+ and HCO3– (Figure 51.11B). The HCO3– is reabsorbed into the interstitial fluid. The NH4+ is transported into the tubule fluid and combines with Cl–, which is excreted in the urine. This process results in the addition of a new HCO3– ion to the blood. The NH4+ is transported into the tubules by means of an NH4+ transporter.