Aquatic invertebrates are either ionic conformers or regulators
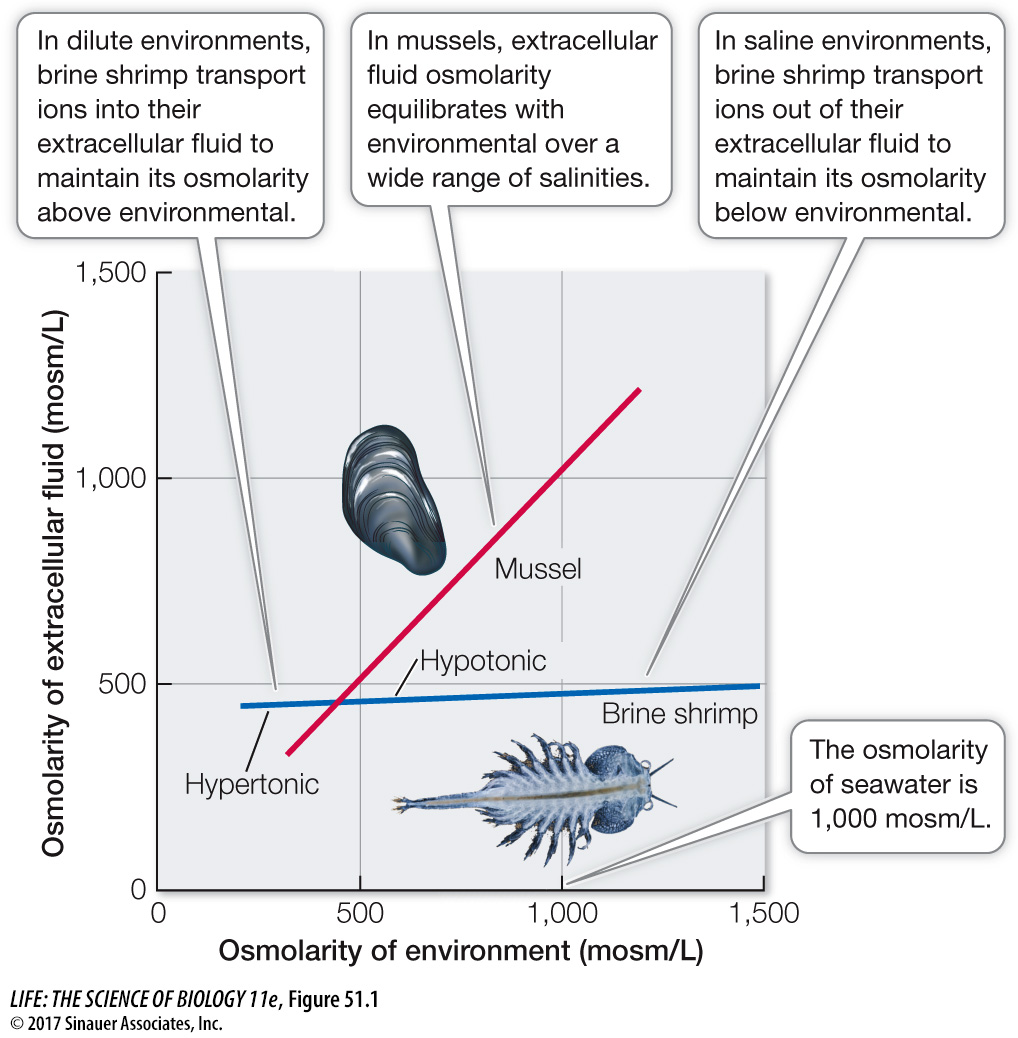
Most invertebrates that live in seawater conform to the osmotic concentration of their environment over a fairly wide range of salinities and are therefore called osmoconformers (Figure 51.1). The osmolarity of seawater in the open ocean is about 1,000 milliosmoles/liter (mosm/L), but it can vary quite a bit in estuaries where it is diluted by an influx of fresh water or in evaporating tide pools as the salt gets concentrated. Osmoconformity can result in considerable energetic savings, as it costs metabolic energy to move ions across membranes to achieve osmotic regulation.
1095
Osmoconformity is not an option for freshwater animals. No cell can do without ions or nutrients, so freshwater animals always have to expend energy to conserve salts and excrete water.
Some marine invertebrates maintain a rather constant osmolarity of their extracellular fluids as the osmolarity of the environment changes, and these animals are therefore called osmoregulators (see Figure 51.1). Osmoregulators in the marine environment mostly maintain their osmotic concentration considerably below that of the environment and are therefore engaged in hypotonic regulation. Occasionally, however, seawater is diluted by an influx of fresh water, as in estuaries, and animals must maintain their osmotic concentrations above that of the environment—
The brine shrimp Artemia illustrated in Figure 51.1 is an osmoregulator with an enormous range of tolerances. Artemia are found in huge numbers in extremely salty environments, such as Utah’s Great Salt Lake and in coastal evaporation ponds where salt is concentrated for commercial purposes (see Figure 25.17) and can reach an osmolarity of 2,500 mosm/L. No animal could survive with internal osmolarities that high; such a solute concentration would cause proteins to denature. Artemia are able to exploit these environments because of their ability to regulate hypotonically by actively transporting NaCl from their extracellular fluid out across their gill membranes to the environment. Artemia cannot survive in fresh water, but they can live in dilute seawater by reversing the direction of transport of NaCl across their gill membranes to maintain the osmolarity of their extracellular fluids above that of the environment, thus becoming hypertonic regulators.
Invertebrates that are osmoconformers still have to be ionic regulators with respect to certain ions. Although the concentrations of Na+ and Cl– in their extracellular fluids may be the same as in seawater, many other ions are regulated at different levels. For example, the concentration of K+ is usually higher, and the concentrations of Mg2+ and of SO42+ are usually lower, in the extracellular fluids than in the seawater. Active transporters maintain the regulated ions in the extracellular fluid at optimal concentrations.