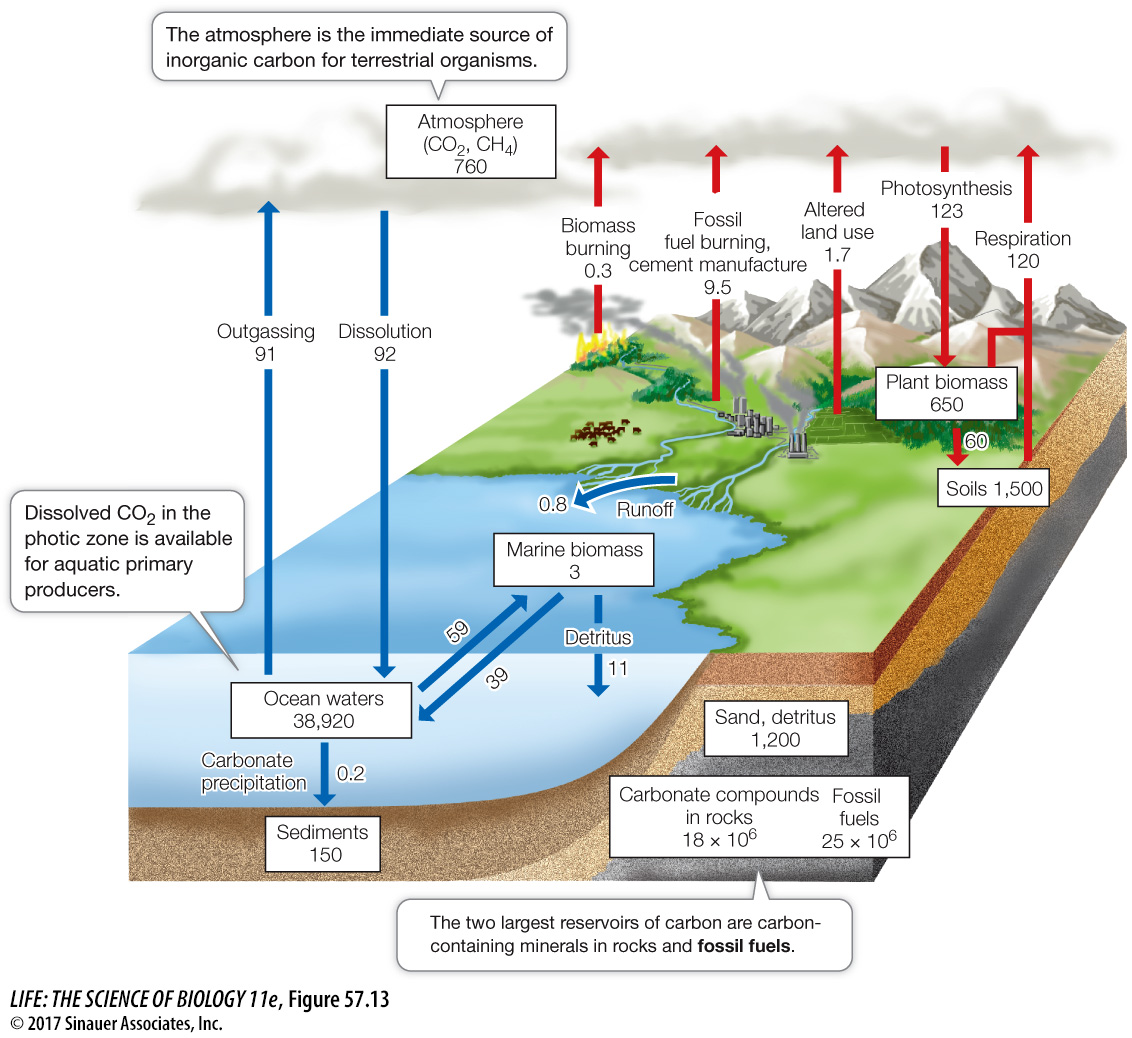
The carbon cycle is being altered by human activities, resulting in climate change
1238
All of the important macromolecules that make up living organisms contain carbon, and much of the energy that organisms use to fuel their metabolic activities is stored in carbon-
Animation 57.2 The Global Carbon Cycle
www.life11e.com/
Over the span of life’s existence on Earth, quantities of carbon were removed from active cycling when organisms died in large numbers and were buried in sediments lacking oxygen. In such anaerobic environments, with few detritivores to reduce organic carbon to CO2, organic molecules accumulate and are eventually transformed into deposits of oil, natural gas, coal, or peat—
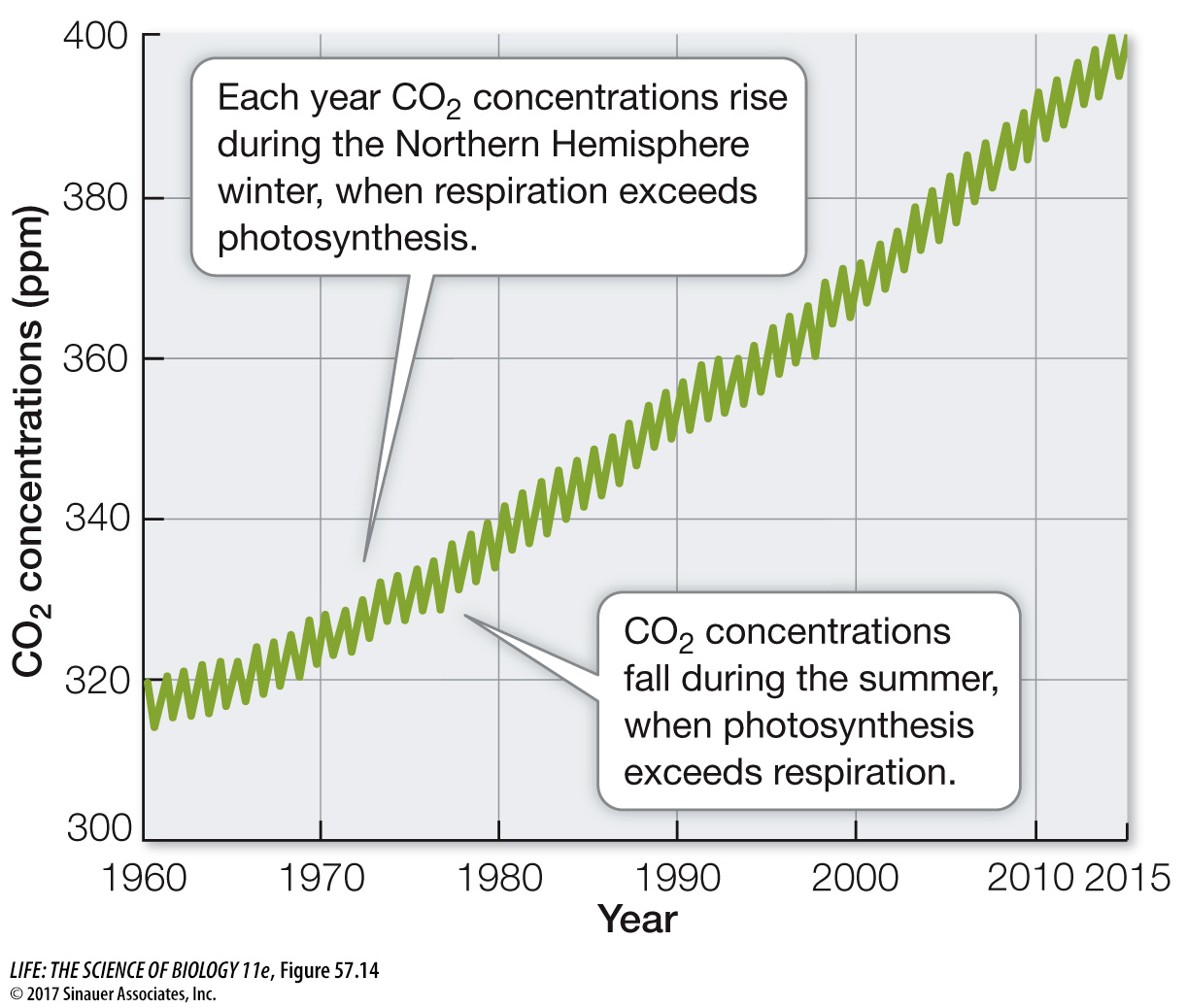
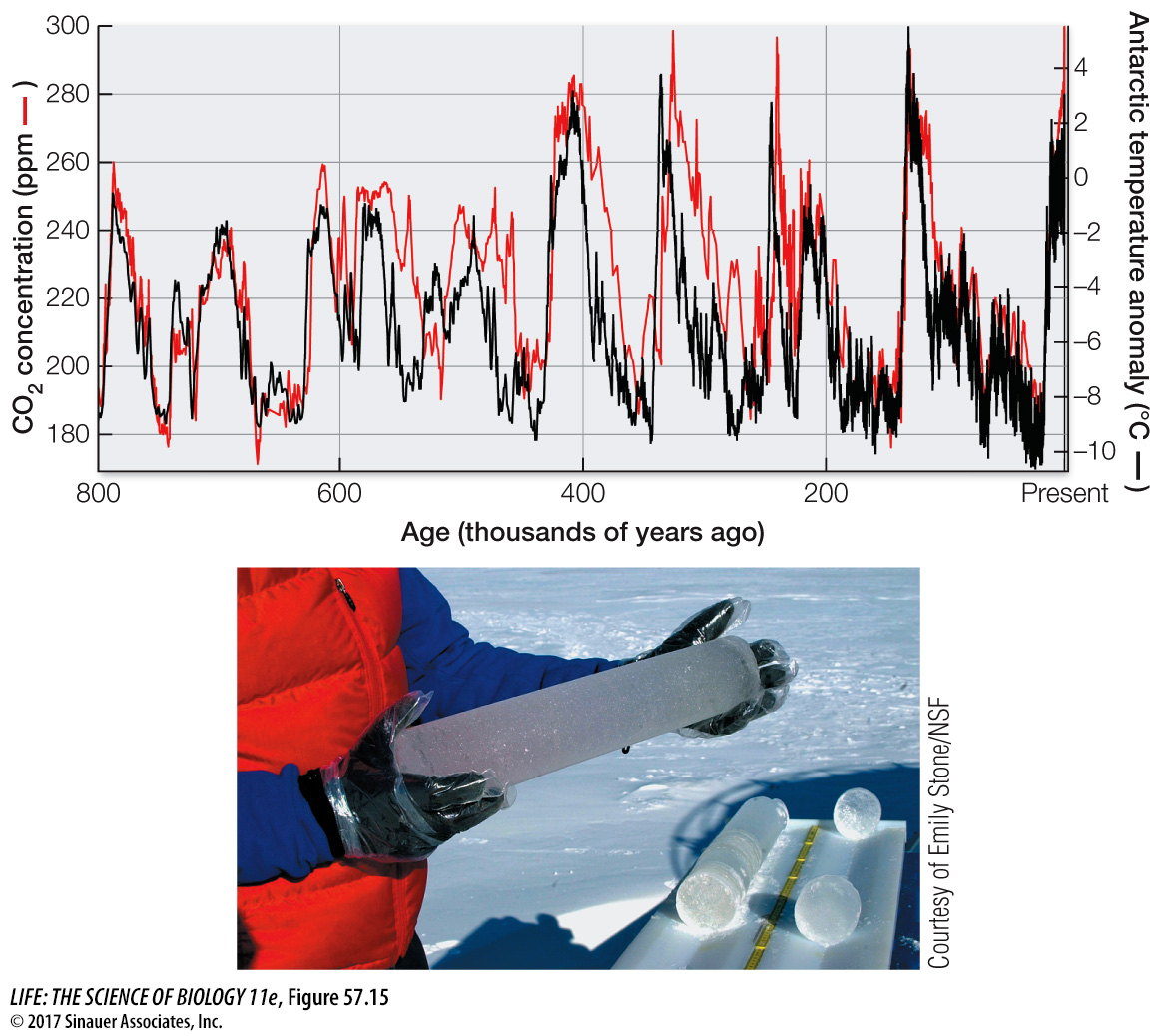
ATMOSPHERIC CO2 AND GLOBAL CLIMATE CHANGE Carbon dioxide is a greenhouse gas, so we would expect increasing atmospheric CO2 concentrations to trap more heat in Earth’s atmosphere and raise temperatures at Earth’s surface (see Figure 53.2). What evidence do we have that this is occurring? Measurements of gases in air trapped in the Antarctic and Greenland ice caps show that temperatures have been warmer when atmospheric CO2 concentrations have been higher and cooler when they have been lower (Figure 57.15). For example, the atmospheric CO2 concentration was very low at the end of the last glaciation (180 parts per million [ppm]), 18,000 years ago, when temperatures were much colder than they are today. In contrast, during a warm interval between 11,000 years ago and the start of the Industrial Revolution (1750), the concentration of CO2 in Earth’s atmosphere was between 260 and 280 ppm. Today atmospheric CO2 concentration is just over 400 ppm (see Figure 57.14), the highest recorded in the last 800,000 years. The increase in CO2 (and two other greenhouse gases, CH4 and N2O) from the burning of fossil fuels has resulted in a roughly 1°C (1.8°F) rise in global temperatures compared with those from 1981 to 2010 (a comparison known as a “temperature anomaly”).
How global climates and ecosystems will change in response to this rapid warming is a subject of intense investigation. Global warming has already resulted in the shrinking of Arctic sea ice, which is currently the lowest ever recorded. As temperatures continue to rise and glacial ice melts, sea level rises (because of both thermal expansion of ocean waters and the addition of glacial meltwater), increasing flooding of coastal cities and agricultural lands, especially during extreme storm events. Nearly one-
1239
Even though atmospheric CO2 is increasing as the result of burning fossil fuels, less than half of the CO2 released into the atmosphere by human activities remains in the atmosphere. Where does the rest of the CO2 wind up? Much of it is absorbed by the oceans in inorganic forms. Over decades to centuries, the oceans exert a large influence over atmospheric CO2 concentrations. Of the CO2 absorbed by the ocean, some is used in photosynthesis by phytoplankton in the surface waters. These organisms remove dissolved CO2 from water, thereby increasing the rate at which atmospheric CO2 is absorbed by surface waters, but as you saw earlier, there are limits to this absorption given nutrient limitation in vast parts of the open ocean. In addition, many marine organisms (including clams, oysters, corals, and planktonic foraminiferans) incorporate carbon in their shells and other structures in the form of calcium carbonate (CaCO3), which is synthesized by combining bicarbonate ions (HCO3–) and calcium ions (Ca2+) dissolved in seawater. When these organisms die, those shells and their embedded carbon sink to the ocean floor.
Today’s oceans absorb millions of tons of CO2 from the atmosphere each day—
1240
*connect the concept Coral bleaching occurs when corals are exposed to warm and/or acidic waters, causing expulsion of their photosynthetic algal symbionts, as described in Chapter 1. These symbionts can sometimes be reacquired, as described in Investigating Life: Can Corals Reacquire Dinoflagellates Lost to Bleaching in Chapter 26.
But as you read in the opening story, the effects of CO2 and ocean acidification vary depending on the organisms or ecosystems considered. Investigating Life: Food Webs in an Acidic and Warming Ocean describes research in Swedish by marine ecologists interested in the effects of ocean acidification and warming on an ecologically critical but predominately primary-
In terrestrial ecosystems, photosynthesis, principally in tropical forests, typically absorbs about the same amount of carbon that is released by terrestrial plants, microbes, and animals through metabolism. In more recent times, as atmospheric CO2 increases, the photosynthetic consumption of CO2 exceeds its metabolic production, which means Earth’s terrestrial vegetation is storing carbon that would otherwise be increasing atmospheric CO2 concentrations—