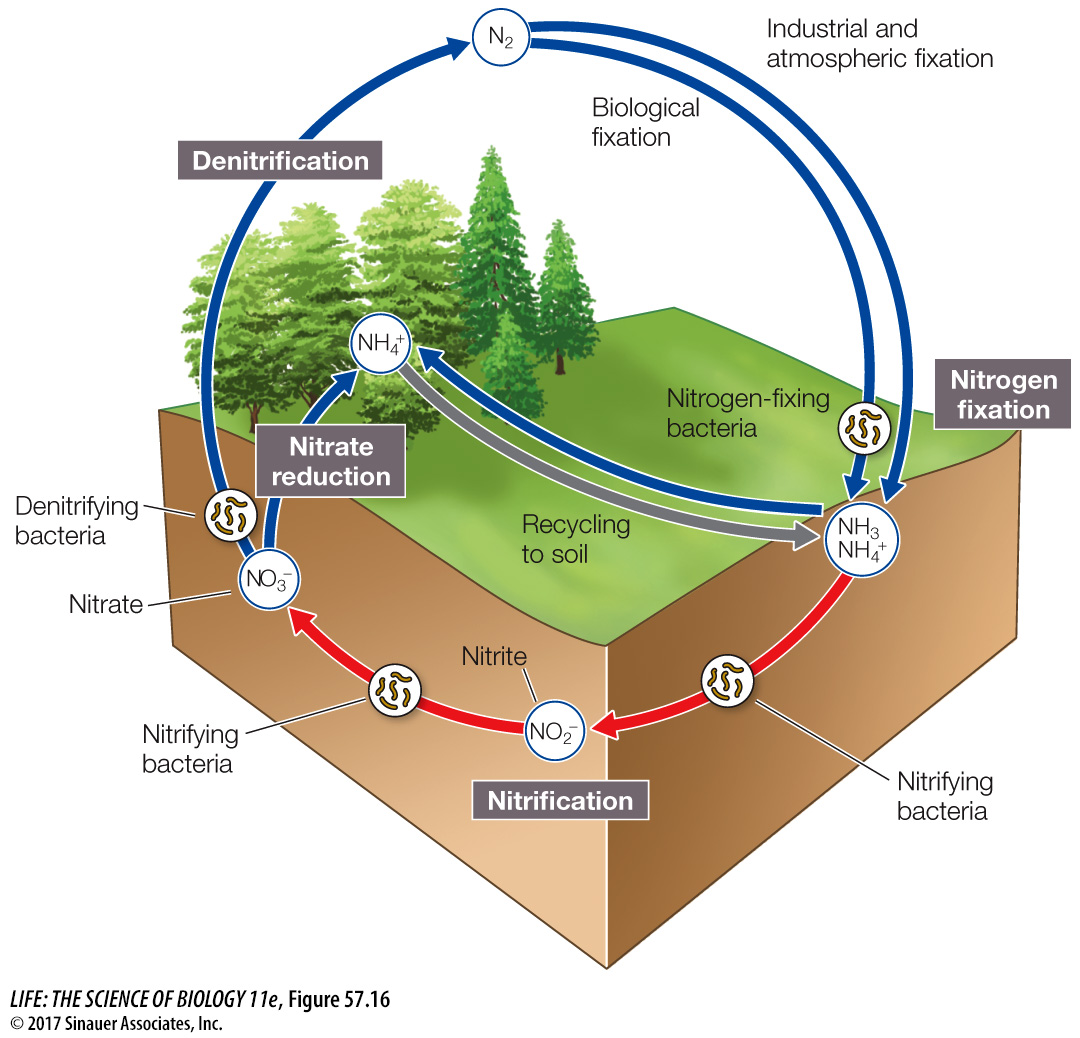
The nitrogen cycle is dominated by biotic processes
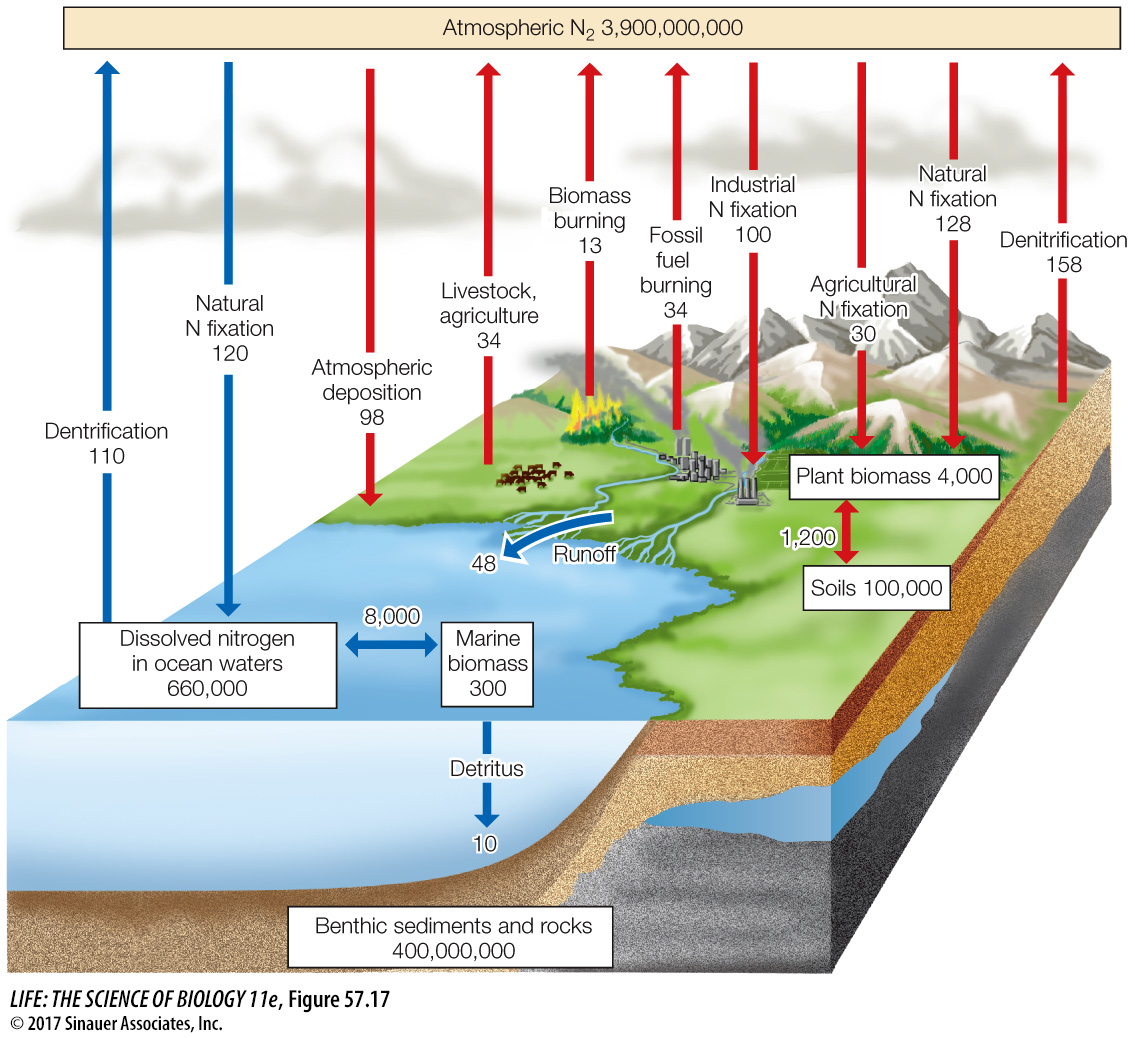
Nitrogen gas is the most abundant gas in Earth’s atmosphere, but most organisms cannot use nitrogen in its gaseous form. Nitrogen enters the system as atmospheric N2 and is fixed by bacteria into ammonia (NH3) (Figure 57.16). Ammonia is rapidly transformed into ammonium (NH4+), which can then be used by plants and bacteria. Nitrifying bacteria can transform ammonium to nitrite (NO2–) and then nitrate (NO3–), both forms of nitrogen that plants and bacteria can use. Denitrifying bacteria take nitrate and convert it back to N2 and N2O gases, which are then released back into the atmosphere. Along the way, plants and bacteria incorporate the nitrogen into their tissues, making it available to higher trophic levels as secondary production and eventually to decomposers. Collectively this microbial processing of nitrogen accounts for about 95 percent of all natural nitrogen flux on Earth, making it a mostly biologically driven cycle (Figure 57.17).
Question
Q: How does the industrial fixation of nitrogen (as a percentage) compare with the other forms of fixation on land?
Industrial fixation of nitrogen accounts for 30 Tg of the 80 Tg total nitrogen fixed, or 37.5 percent.
Animation 57.3 The Global Nitrogen Cycle
www.life11e.com/
All living organisms require nitrogen, and the inability of the vast majority of organisms to use N2 means that usable nitrogen is often in short supply. Populations of nitrogen-
Human activities that fix nitrogen, such as the manufacture of artificial fertilizers, have had large and unanticipated effects on the nitrogen cycle. The extensive use of artificial fertilizers on agricultural crops, coupled with the burning of fossil fuels (which generates nitric oxide and nitrogen dioxide), has resulted in total nitrogen fixation by humans being nearly equal to global natural nitrogen fixation (see Figure 57.17). This human-
1241
investigating life
Food Webs in an Acidic and Warming Ocean
experiment
Original Paper: Alsterberg, C., J. S. Eklöf, L. Gamfeldt, J. N. Havenhand and K. Sundbäck. 2013. Consumers mediate the effects of experimental ocean acidification and warming on primary producers. Proceedings of the National Academy of Sciences USA 110: 8603–
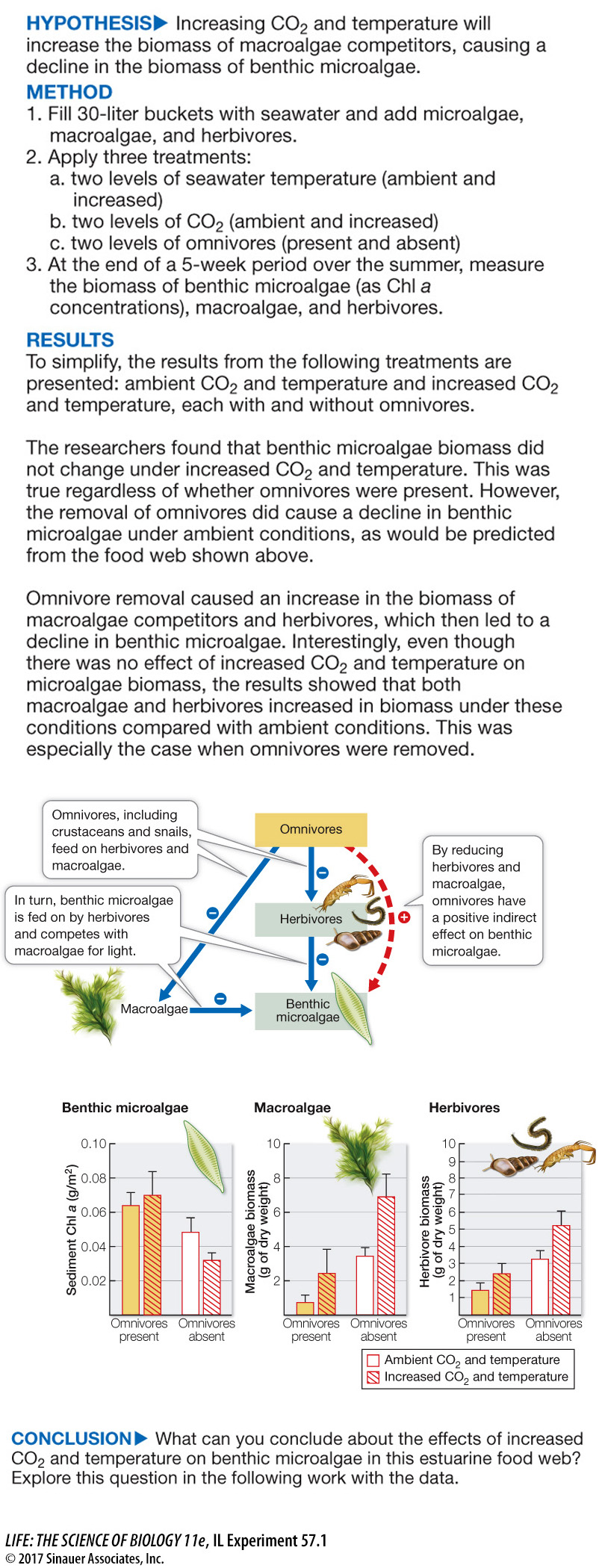
Christian Alsterberg and his colleagues considered how the food web in an estuarine ecosystem on the western coast of Sweden was influenced by ocean acidification and warming. The researchers chose to focus on benthic microalgae (single-
work with the data
To interpret the Alsterberg et al. study, we consider why benthic microalgae biomass did not change under increased CO2 and temperature within the context of the experimental manipulation of the food web.
QUESTIONS
Question 1
Draw two food webs for this system, one with and one without the omnivores. Use arrows to indicate the direct (solid) and indirect (dashed) interactions and symbols to indicate negative (–) or positive (+) interactions.
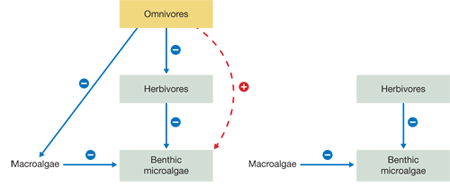
Question 2
Now, using data from the graphs in the experiment, modify the two food webs to show how increased CO2 and temperature will affect the strength and direction of the food web interactions. Vary the width of the arrows to indicate the relative strength of the interactions, and use symbols to indicate whether the interactions are negative (–) or positive (+). Indicate whether benthic microalgae increase, decrease, or stay the same compared with ambient conditions.
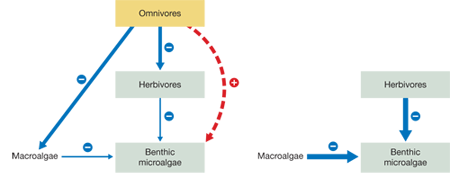
Under increased CO2 and temperature, macroalgae and herbivores increase in biomass. When omnivores are present, this greater biomass will be utilized by omnivores, resulting in a stronger negative interaction between them and their food sources. The stronger negative interaction will lead to a stronger indirect positive effect for benthic microalgae, which will not change in their biomass as a result. However, when omnivores are absent, macroalgae and herbivores will not be kept in check, and their negative interactions with benthic microalgae will grow even stronger, resulting in the decline of benthic microalgae.
Question 3
Using the food webs you drew for the answer to Question 2 as your guide, explain why increased CO2 and temperature had no effect on microalgae biomass compared with the ambient conditions.
The food webs and supporting data in the graphs show that with increased CO2 and temperature, macroalgae and herbivores both increased in biomass, causing negative effects for benthic microalgae (the biomass declined). However, these negative effects for benthic microalgae did not manifest themselves when omnivores were present. By keeping macroalgae and herbivores in check under increased CO2 and temperature, omnivores indirectly benefited benthic microalgae and allowed them to maintain the same biomass as that seen under ambient conditions.
Question 4
Under increased CO2 and temperature, what would happen to benthic microalgae biomass if the omnivores ceased feeding on macroalgae and just preyed on herbivores?
If omnivores ceased feeding on macroalgae, benthic microalgae would decline in biomass because their competitors would increase. But the decline in biomass would be less than if these top consumers were removed altogether. The omnivores would still feed on the herbivores, creating a trophic cascade that would benefit benthic microalgae.
A similar work with the data exercise may be assigned in LaunchPad.
One consequence of artificial fertilizer production is an increase in the greenhouse gas nitrous oxide (N2O), resulting in more tropospheric ozone (O3) and smog. Trophospheric ozone is itself a greenhouse gas and thus can contribute to global climate change.
Some of the nitrous oxide that enters the atmosphere falls back to land in precipitation or as dry particles. This deposition of nitrogen from the atmosphere has increased dramatically during recent decades. Nitrogen deposition could affect the composition of terrestrial vegetation by favoring those plant species that are best adapted to take advantage of high nutrient levels, and which may then outcompete other species.
1242
Another consequence of nitrogen fertilizers is eutrophication, a process we considered in Key Concept 57.2 for phosphorus additions in lake ecosystems. In this case, when more nitrogen fertilizer is applied to agricultural lands than can be taken up by the crops, the excess nitrogen moves out of the system in surface runoff, or downward into groundwater, and ultimately ends up in rivers, lakes, and oceans. So-
Media Clip 57.1 Tracking Dead Zones from Space
www.life11e.com/