Chapter 9
RECAP 9.1
A complex chemical transformation occurs in a series of separate reactions that form a metabolic pathway. Each reaction is catalyzed by a specific enzyme. Many metabolic pathways are similar in all organisms, from bacteria to humans. In eukaryotes, many metabolic pathways are compartmentalized, with certain reactions occurring inside specific organelles. Some key enzymes in each metabolic pathway can be inhibited or activated to alter the rate of the pathway.
NAD+ is a reducing agent (it accepts electrons), and O2 is an oxidizing agent (it donates electrons).
It is an oxidation (removal of H from C2 and C3 of succinate).
It is exergonic, because it is an oxidation.
It requires the redox coenzyme NAD or FAD.
RECAP 9.2
In glycolysis, the net yield is 2 ATP per glucose and 1 NADH per glucose.
Pyruvate oxidation produces the two-
carbon molecule acetyl CoA, which is activated and then participates as a first substrate in the citric acid cycle. The citric acid cycle regenerates the four-
carbon acceptor molecule for the next acetyl CoA from pyruvate. Glycolysis does not regenerate a starting material.
RECAP 9.3
A series of electron carriers in the inner mitochondrial membrane transports electrons by reduction–
oxidation. As the electrons are added to each carrier, protons are transported via the carrier into the intermembrane space. The experiment in Figure 9.9 shows that in the absence of electron transport, a gradient of protons across the membrane is sufficient to produce ATP if the ATP synthase is present in the membrane. The experiment in Figure 9.10 shows directly that ATP synthase can carry protons from a gradient, harnessing the potential energy from the gradient to make ATP.
If cytochrome c remains reduced and cannot accept electrons, the electron transport (respiratory) chain stays reduced and NADH and FADH2 remain reduced. This prevents oxidation reactions in the citric acid cycle and pyruvate oxidations, so pyruvate cannot be converted to acetyl CoA. Instead, pyruvate is converted to lactic acid, regenerating some NAD that can be used so that glycolysis can continue. Because the electron transport chain is not working, no proton gradient is set up in the mitochondria, and ATP is not made by oxidative phosphorylation.
If antimycin A were present, it would make no difference to the results of the experiment, since an artificial proton gradient was already set up.
RECAP 9.4
NAD+ is an electron acceptor for reactions in glycolysis and pyruvate oxidation. If it is not present in its oxidized form, the oxidation of these substrates in the two reactions does not occur, and the pathways stop.
Fermentation replenishes NAD+ by oxidizing NADH during chemical oxidations forming lactate or ethanol from pyruvate.
RECAP 9.5
A catabolic interconversion of a lipid: Fatty acids are catabolized to acetyl CoA. This enters the citric acid cycle and is oxidized to CO2, providing reduction energy to form NADH and FAHD. An anabolic interconversion of a protein: Amino acids are converted to intermediates in glycolysis and the citric acid cycle. These intermediates can be used in anabolism to form glucose, fats, or other amino acids to make proteins.
Phosphofructokinase acts early in glycolysis and is a focal point for turning off or activating the rest of the energy pathways. It is turned off by later products (ATP, citrate) and turned on when energy is needed (ADP).
Some amino acids are converted to intermediates of glycolysis. Once they enter glycolysis, these intermediates are further metabolized to a glycolytic intermediate that can be converted to glycerol, which is incorporated into triglycerides. Glycolysis and pyruvate oxidation produce acetyl CoA, which is converted to fatty acids and incorporated into lipids. Glucose is converted in glycolysis to acetyl CoA, which is then converted to fatty acids as above.
WORK WITH THE DATA, P. 183
Both groups of mice gained weight as they aged, but the mutant mice gained less weight than the normal mice.
The amount of food eaten and the levels of exercise were the same for both groups. So the lower weight gain in the mutant mice was not due to food intake or exercise.
UCP1 levels were much higher in the mutant mice than in the normal mice. The correlation between higher UCP1 and lower weight gain indicates that uncoupling of mitochondrial ATP synthesis from electron transport in brown fat may be responsible for lowered weight gain.
A-
FIGURE QUESTIONS
Figure 9.3 The H atom comes from the oxidation of a substrate.
Figure 9.7 –20 kcal/mol
Figure 9.13 DNA can be an energy source. When DNA is hydrolyzed, the nucleotides can be metabolized into intermediates in the citric acid cycle. The intermediates are then oxidized and the energy released to reduced coenzymes, which release their energy when oxidized in the mitochondria to form ATP. However, because it is the genetic material and must be preserved, DNA is protected from hydrolysis after it is made by being sequestered in the cell nucleus.
APPLY WHAT YOU’VE LEARNED
The body first uses glucose supplied from the last meal, then begins to break down glycogen. Once all of the glycogen is gone, the body makes glucose through gluconeogenesis using molecules present in the body.
Mammals break down proteins and use amino acids to make glucose through gluconeogenesis. Mammals also use glycerol from triglycerides, but they do not use fatty acids in this way. Fatty acids do not stimulate gluconeogenesis.
The person will lose muscle mass because proteins in muscle cells will be broken down to make glucose via gluconeogenesis. This is not advisable because this will weaken the person’s body and put him or her in a state of low body fitness. People generally do not realize this because they think that dieting will cause loss of fat rather than muscle.
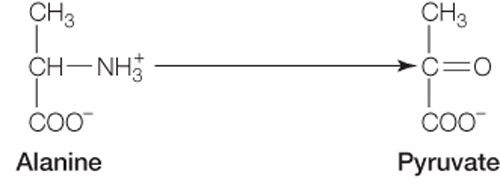
Any of the carbon atoms in alanine could be labeled with carbon-
14 to trace it through the gluconeogenesis pathway, since only the NH3 group is removed from the alanine carbon chain.