Chapter 2
RECAP 2.1
Elements with the same number of valence electrons are placed in the same vertical groups in the periodic table. For example, the electrons in sodium occupy the following shells (in order from the nucleus outward): 1s2, 2s2, 2p6, 3s1. Having one electron in the valence shell places sodium in Group 1. Lithium and potassium, which likewise have one electron in the valence shell, are in Group 1 as well.
An atom is stable when its eight electrons occupy its outermost shell (with the exception of hydrogen, which is stable with two electrons). In bonding, atoms either share electrons with, or donate to or receive electrons from, another atom to achieve stability.
The human body has the same elemental composition as Earth’s crust but in very different proportions.
RECAP 2.2
In a covalent bond, the shared electrons between two atoms are actually part of both atoms. They are close together, and it takes a lot of energy to break them apart. In an ionic attraction, the electrons are transferred and the two atoms are relatively far apart, so it takes less energy to break an ionic attraction.
nonpolar; polar; polar; nonpolar
hydrophobic; hydrophilic; hydrophilic; hydrophobic
C═O: δ– at O; O—
P: δ – at O
This is an example of van der Waals forces, which act over a short distance and do not involve polarity.
RECAP 2.3
X C6H12O6 + X O2 → X CO2 + X H2O
In the burning of propane, there is a chemical transformation. Some chemical energy is released as heat and light.
RECAP 2.4
A solution contains a solvent (e.g., water) and a substance dissolved in the solvent (e.g., table salt, NaCl). Water is the medium of life because most molecules in living organisms either dissolve in water or interact with it.
A 1 molar (1 M) solution has a concentration of 1 mole per liter, which means it has 6.02 × 1023 molecules per liter. A 10–8 M solution has 6.02 × 1015 molecules per liter. If there is 10–6 liters, the number of molecules is 6.02 × 109.
This reaction increases the H+ concentration in the blood and thereby decreases the pH. To increase the pH, and thereby decrease the H+, the reverse reaction could be used as a buffer:
H+ + HCO3– → CO2 + H2O
WORK WITH THE DATA, P. 25
The average local ratios differ from the all-
countries average ratio. To test the hypothesis that the ratios are significantly different, a chi- square test could be used. A graph of the data shows an increasing 13C:12C ratio as latitude away from the equator increases. This implies that different plants occur in different regions. Animal feeds used locally tend to be composed of plants grown locally.
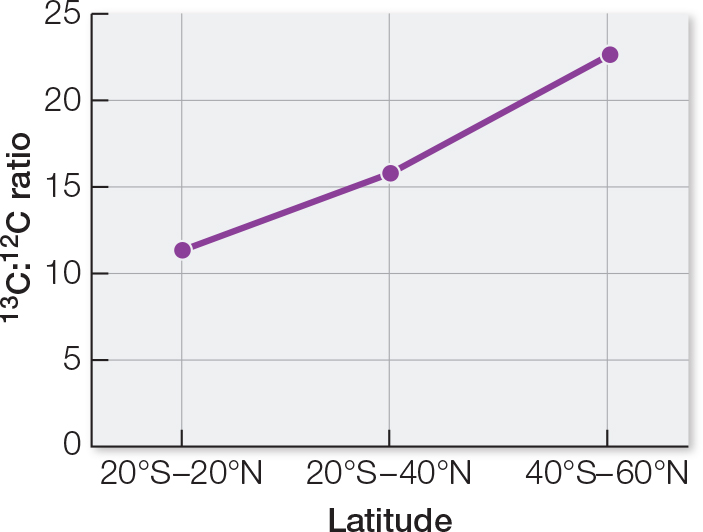
FIGURE QUESTIONS
Figure 2.3 The technique could be used to monitor therapy. As therapy progresses and the patient improves, one would expect that the brain region involved would show more activity.
Figure 2.6
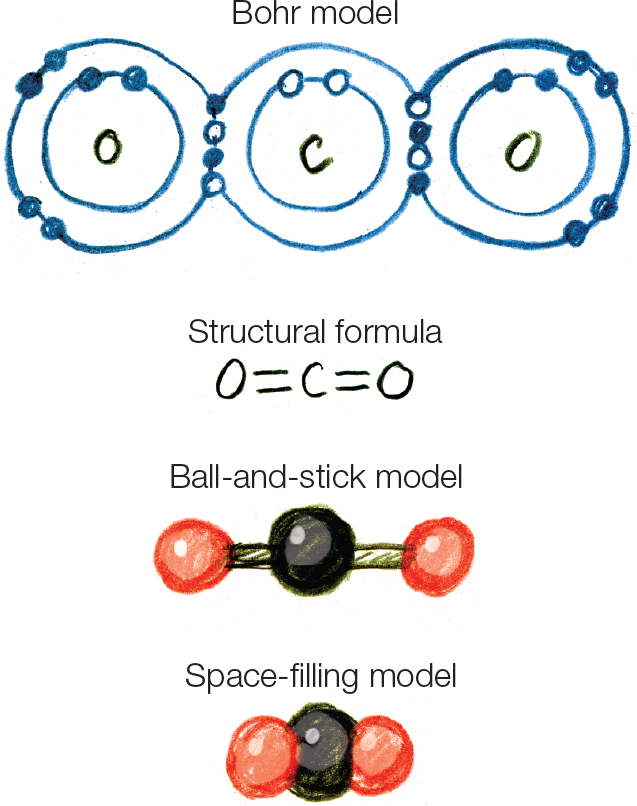
Figure 2.8 Ca (atomic number 20) has two valence electrons. Cl (atomic number 17) has seven valence electrons. So combining, two electrons are lost from one Ca atom to two atoms of Cl: formula CaCl2.
Figure 2.9 At the chemical level, evaporation eliminates water for hydration of the ions, so the ions are no longer separated. At the physical level, the salt no longer dissolves and crystals form.
Figure 2.10 Heat will break hydrogen bonds. Because these bonds affect the interactions of some chemical groups with other groups at different locations in the molecule, the three-
APPLY WHAT YOU’VE LEARNED
HCO3– + H+ ⇌ H2CO3
The pH would not change. As H+ was added due to ionization of acetoacetic acid, it would push the equilibrium of the above reaction to the right, as shown below.
HCO3– + H+ → H2CO3
added H+ reacts with HCO3– to shift equilibrium to the right
That is, bicarbonate ions would attach to the added H+, so the overall free H+ would not change.
Eventually the bicarbonate buffer system would be overwhelmed. It would no longer be able to take up the additional H+ ions building up in the blood and shuttle them into carbonic acid. At that point, H+ ions would accumulate, which would cause the blood pH to drop. The body would not be able to maintain a constant pH and its cells would be exposed to lower than normal pH conditions in the blood. In this case, the person would become severely ill because the cells would be exposed to an abnormal environment that then disrupts normal cell functions.
A-
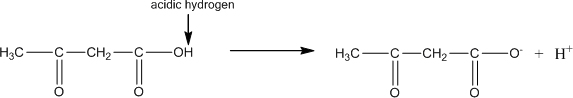
4 Acetone does not contain an acidic hydrogen, so it would not be expected to affect any acid or base buffering systems in the blood.
Four test possibilities are: insulin level (lower than normal), blood glucose level (elevated), blood pH (lower than normal), and bicarbonate level (lower than normal).