Chapter 3
RECAP 3.1
Structural isomers have the same number of atoms, but they are arranged differently. Cis-
trans isomers typically have two double- bonded carbon atoms, and each of the two carbons is covalently bonded to two different groups as well (e.g., to −H, –CH3, or another attached group). When the two substituents are oriented in the same plane relative to the double bond, the isomers are cis. When the two groups are oriented in opposing directions, the isomers are trans. Optical isomers have carbon atoms with four different atoms or groups bonded to them, and this makes two mirror images possible. Mannose and galactose have the same atomic formula, C6H12O6, but the arrangement of atoms is different. Compare carbons 2 and 4. These sugars have the hydroxyl (–OH) functional group. Its polarity helps the sugars dissolve in water. The –OH group also can participate in bonding the sugar to other molecules through condensation reactions.
H-
A- OH + H- B- OH → H- A- B- OH + H2O H-
A- B- OH + H- C- OH → H- A- B- C- OH + H2O
RECAP 3.2
When one amino acid (such as lysine) is replaced by another, the primary structure of the protein is altered. The change could affect tertiary structure if the protein folds differently as a result of electrostatic attractions between charged amino acids (+ to –). In this example, a negatively charged amino acid (aspartic acid) has taken the place of a positively charged one (lysine), and this may prevent correct folding, particularly if a negatively charged amino acid elsewhere in the polypeptide chain is involved in folding (it is attracted to a + amino acid). The same forces might be at work in the interaction of separate chains for quaternary structure.
The observations support explanation “a.” Glycine is small and nonpolar. Glutamic acid and arginine are larger and polar (charged). Serine and alanine are small: the protein retains its shape. But serine is polar (it has –OH as its R group), and that does not affect the structure. Valine is larger and nonpolar, and this affects shape. So the issue is size.
See Figure 3.10. Heat breaks hydrogen bonds and other weak interactions that maintain protein shape. Disulfide bonds also are required for normal protein shape. Styling and perms partially denature keratin, then renature the protein in a new shape. Your investigation might involve measuring keratin protein structure of hair before and after disrupting hydrogen bonds and disulfide bonds.
RECAP 3.3
Starch and glycogen have many carbon atoms bonded covalently to one another. These covalent bonds require energy for formation and release energy when broken.
The many hydrogen bonds between polysaccharide chains of cellulose hold them together, and the covalent bonds within the chains are strong individually.
RECAP 3.4
The phospholipids would aggregate, with the polar heads facing in and the tails facing out—
the reverse of what happens in membranes. This would probably be aggregates, rather than a bilayer. Fats are solid at room temperature and have single bonds (saturated) between fatty acid carbon atoms (saturated). Oils are liquid at room temperature and have double bonds between some fatty acid carbon atoms (unsaturated).
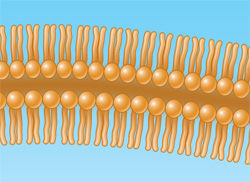
Steroids and some vitamins are classified as lipids because they are composed largely of carbon atoms linked together with substituent hydrogen atoms and are insoluble in water and soluble in nonpolar solvents.
WORK WITH THE DATA, P. 44
The genetically engineered silk was better than the native spider silk. It was thicker, required more force to break, and resisted strain better than native spider silk.
Fiber thickness is reported because it is one indicator of fiber strength.
The t-test could determine this.
Spider silk is stronger than Kevlar or steel and weighs less.
WORK WITH THE DATA, P. 52
Disulfide bonds began forming almost immediately after reoxidation began. Enzyme activity began appearing 100 minutes after reoxidation began. There are two reasons for the delay between the beginning of disulfide bond formation and the reappearance of enzyme activity. First, there are four disulfide bonds in the protein, all of which have to re-
form before enzyme activity is restored. In other words, the first disulfide bonds to form aren’t sufficient to restore activity, so there is a lag before activity reappears. Second, there are other chemical interactions, such as hydrogen bonding and hydrophobic interactions that occur after the protein has initially folded due to disulfide bond formation and which are also necessary for enzyme activity. The absorption peak for the native protein was at about 278 nm; the peak for the reduced (denatured) protein was at about 275 nm. Reoxidation resulted in a return to the native spectrum. Under the denaturation conditions of these experiments, as long as the primary structure of RNase A is retained, the proper environmental conditions will result in a return to the native structure and a fully functional molecule.
FIGURE QUESTIONS
Figure 3.7 Primary structure will be unaffected, because it is held together by strong covalent bonds.
Figure 3.9 Regions in lysozyme that face the outside (water) environment are hydrophilic, whereas those on the inside are generally hydrophobic.
Figure 3.12 Weak interactions shown within or between molecules require a relatively low amount of energy to break them apart.
Figure 3.14 Heat shock proteins protect cellular proteins from being denatured and possibly broken down. This can occur not only when proteins are exposed to heat, but in any chemical situation where their structure is altered (e.g., a change in pH).
APPLY WHAT YOU’VE LEARNED
The forces involved are noncovalent and involve mainly hydrophobic and van der Waals interactions, since the R groups tend to be hydrophobic groups (Val, Ile, Phe, Gly). The R groups of these amino acids could stick out from the insulin polypeptide backbone so as to interact with its target proteins to form these noncovalent interactions.
These amino acids are invariant across all vertebrate species shown in the table, suggesting that they are essential in maintaining insulin’s biological activity.
Certain changes are allowed as long as the overall tertiary structure of the protein is not changed significantly. Only amino acids that are similar in structure and properties would be able to function in a similar way at any position.
Cysteine residues are likely to be essential because they form disulfide bridges, linking the two chains together and stabilizing the protein’s tertiary structure. No other amino acid can fulfill this role. We could compare these amino acids across the same vertebrate species shown above to see whether any have other amino acids at the cysteine locations.