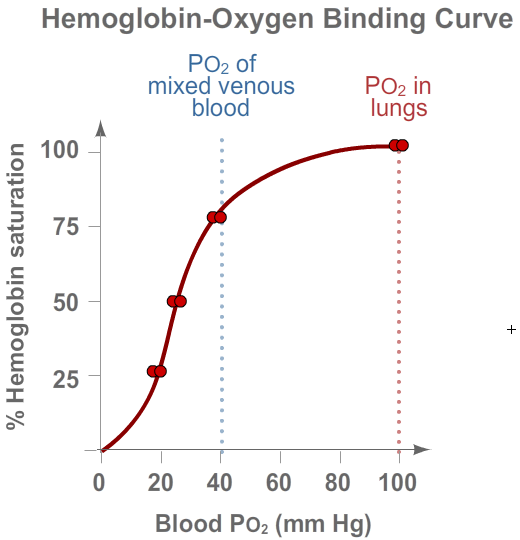
Click the buttons below to see how these factors influence the binding of O2 to hemoglobin.
Carbon Monoxide
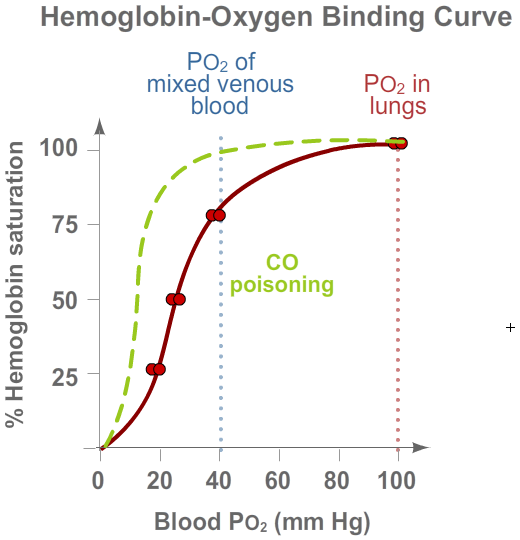
Carbon monoxide (CO) poisoning shifts the hemoglobin-O2 binding curve far to the left.
CO has a much greater affinity for hemoglobin than does O2 (230 times!). Thus, only a
very small amount of inhaled CO will occupy O2-binding sites on the hemoglobin and
reduce the amount of O2 that the hemoglobin can carry even when it is 100% saturated.
In addition, the CO increases the affinity of hemoglobin for O2 molecules.
Thus, even the O2 being carried by the hemoglobin will not be released to the
tissues at PO2's compatible with life.
Carbon Dioxide
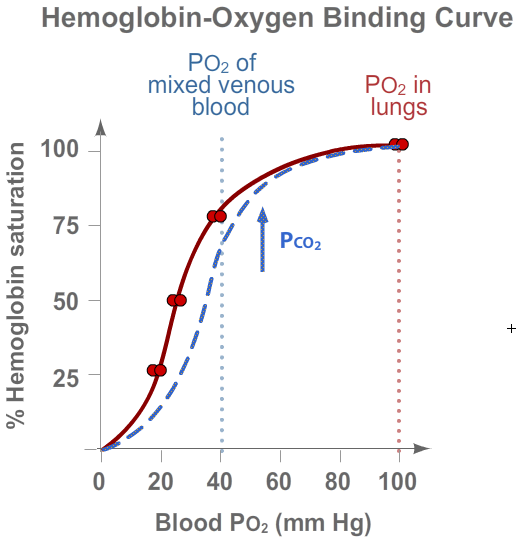
Metabolically active tissues produce a lot of carbon dioxide (CO2).
As more CO2 enters the blood, it starts to bind to hemoglobin (at nonheme sites,
which are sites other than the O2-binding sites). As a result of CO2 binding,
hemoglobin changes its conformation slightly, causing it to have less affinity for O2.
As a result, hemoglobin releases O2 more readily, unloading O2 at the tissues that need it most.
This action of CO2 is depicted in the right-shifted hemoglobin-oxygen binding curve (in blue).
pH
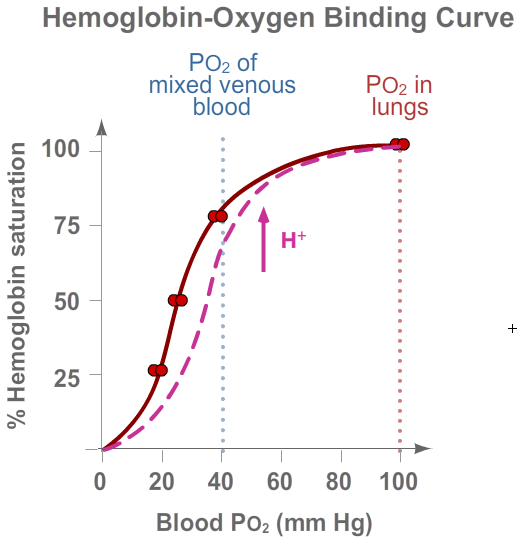
The influence of pH (hydrogen ion concentration) on the function of hemoglobin is known as the
Bohr effect. As blood passes through metabolically active tissue, such as exercising muscle,
it picks up acidic metabolites, such as lactic acid, fatty acids, and CO2.
As a result, blood pH falls. The excess H+ binds preferentially to deoxygenated hemoglobin and
decreases its affinity for O2, and the O2 binding/dissociation curve of
hemoglobin shifts to the right (see pink curve). This shift means the hemoglobin will release
more O2 in tissues where pH is low—another way that O2 is supplied where
and when it is most needed.
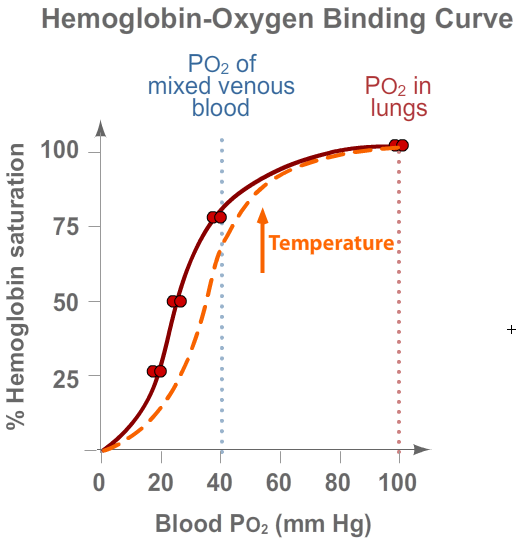
Temperature
Metabolically active tissues generate more heat, which affects the hemoglobin-oxygen binding curve.
As hemoglobin heats up, it reduces its affinity for O2, releasing it to the tissues that
need it most. This action of higher temperatures is depicted in the right-shifted hemoglobin-oxygen
binding curve (in orange).
Exercise and High Altitude
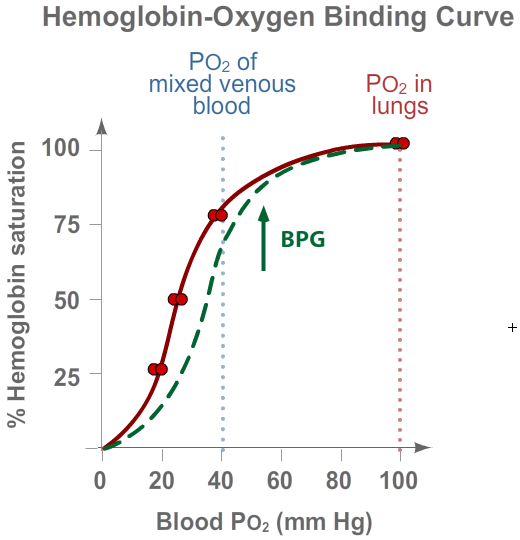
2,3-Bisphosphoglyceric acid (BPG) is a metabolite of glycolysis. Mammalian red blood cells respond to
low PO2 by increasing their rate of glycolysis and thus producing more BPG.
BPG reversibly combines with deoxygenated hemoglobin and lowers its affinity for O2.
The result is that at any PO2, hemoglobin releases more of its bound O2
than it otherwise would. When humans go to high altitudes, or when they cease being sedentary and
begin to exercise, their red blood cells are exposed to a lower PO2 and their
level of BPG goes up, making it easier for hemoglobin to deliver more O2 to tissues.
BPG shifts the O2-binding/dissociation curve of mammalian hemoglobin to the right (see curve in green).

