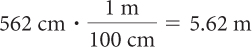SI Units of Measure
A-0
Scientists rely on repeatable measurements as they study the physical world. It is important that they use consistent units of measure worldwide. In 1960 an international council standardized the metric system, creating the Système International d’Unités (International System of Units), abbreviated as SI.
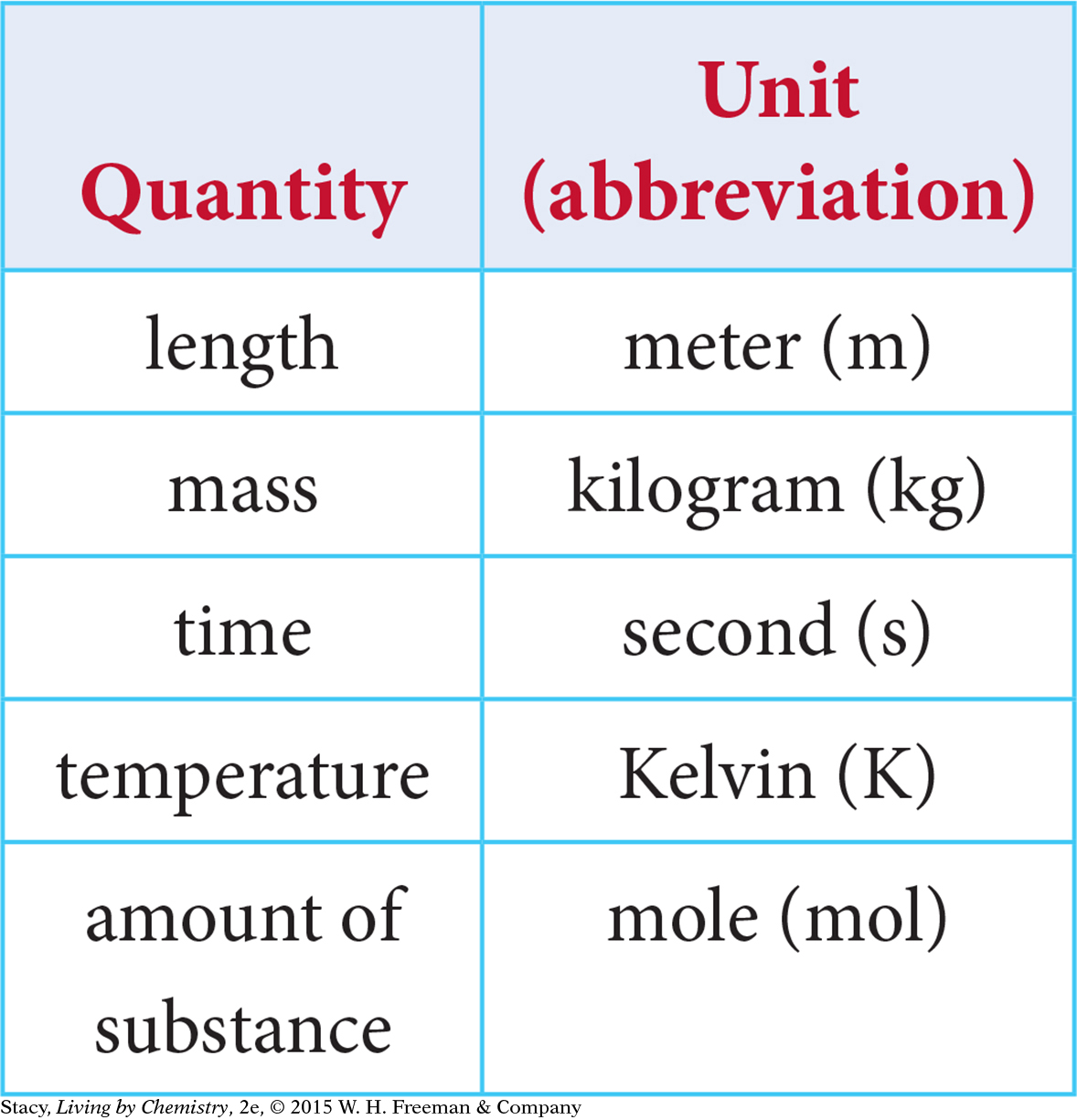
In the table at left, there are the basic SI units used in chemistry. Other units such as density and volume are combinations of these.
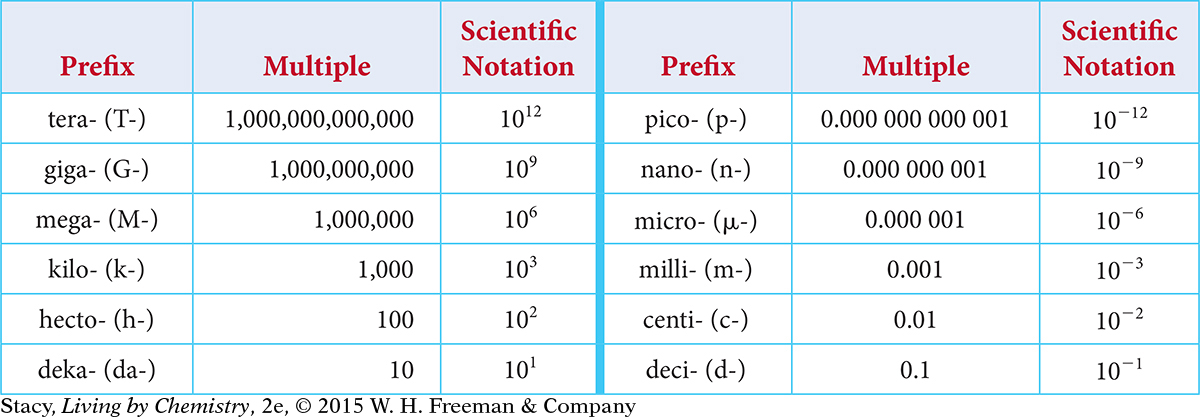
SI units are based on powers of 10. Larger and smaller units get their names by combining standard prefixes with these basic units. For example, the word centimeter is a combination of centi- and meter. A centimeter is one one-hundredth of a meter. These are the prefixes used in the SI, along with their abbreviations and their meanings. (A kilogram is the only basic SI unit that has a prefix as part of its name.)
Example 1
Length Conversions
How many meters does each of these lengths represent?
562 centimeters
2.5 kilometers
Solution
Example 2
The Mass of One Liter
One milliliter, or cubic centimeter, of water at 4 °C weighs 1 g. How much does 1 L of water at 4 °C weigh?
Solution
One milliliter is one one-thousandth of a liter; multiply the mass of 1 mL by 1000 to get the mass of 1 L.
1000·1 = 1000 g =1 kg
Practice Exercises
Convert these measurements to the indicated units.
A-1
7 m = ____cm
3200 mL = ____L
20,012 cm = ____km
0.003 kg = ____g
16 m2 = ____cm2
2 m3 = ____dm3
Answers
700 cm
3.2 L
0.20012 km
3g
160,000 cm2
800 dm3