Averages
Scientists are often interested in the typical result of a repeated experiment. The average, also called the mean, is one way to determine a typical value. The average is calculated by totaling the data values and dividing by the number of values.
Example 1
The Mean
Find the average of these numbers: 14, 23, 10, 21, 7, 80, 32, 30, 92, 14, 26, 21, 38, 20, 35, 21.
Solution
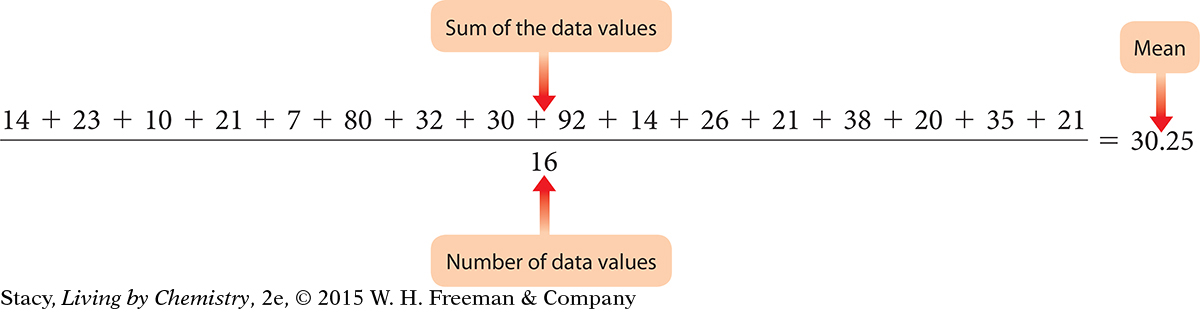
The average is 30.25.
Sometimes the data values are not of equal importance in contributing to the average. You need to weight the values differently.
Example 2
The Weighted Average
On a chemistry quiz, 1 student got 100%, 7 students got 95%, 12 students got 90%, 1 student got 85 %, 5 students got 80%, 3 students got 75%, and 1 student got 70%. What was their average (mean) score?
Solution
One solution would be to change the list so that 95% appeared 7 times, 90% appeared 12 times, and so on. However, it is more efficient to use a weighted average as shown here.
(1)(100) + (7)(95) + (12)(90) + (1)(85) + ( 5)(80) + (3)(75) + (1)(70) = 2625
Thirty students took the quiz, so divide 2625 by 30.
The average score was 87.5%.
Example 3
Average Atomic Mass
The element silver, Ag, has two naturally occurring isotopes. Approximately 52% of all silver consists of atoms with 60 neutrons, and 48% consists of atoms with 62 neutrons. Calculate the average atomic mass of silver atoms.
Solution
Silver atoms have 47 protons. The atoms with 60 neutrons have masses of 47 + 60 = 107 amu. The atoms with 62 neutrons have masses of 47 + 62 = 109 amu. If you have a sample of 100 silver atoms, 52 atoms will have a mass of 109 amu and 48 atoms will have a mass of 109 amu. Use a weighted average. The total mass is 52(107 amu) + 48(109 amu) = 10,796 amu. The average mass is 10,796 amu/100 = 107.96. This is close to the atomic mass of 107.9 amu shown on the periodic table.
Practice Exercises
Find the average of these numbers: 52.3, 18.91, 35.66, 4.35.
A student had these scores on chapter tests: 87%, 90%, 95%, 92%. He got 92% on one unit test and 86% on the other. His final exam score was 91%. Unit tests count twice as much as chapter tests, and the final exam counts four times as much as a chapter test. What is his average score?
About 76% of all chlorine atoms have an atomic mass of 35.00 amu; about 24% have an atomic mass of 37.00 amu. Use this information to calculate the average atomic mass of 100 chlorine atoms.
Answers
27.8
90%
35.48 amu