LESSON 54: Sorry, Charlie: Charles’s Law
282
THINK ABOUT IT
The vent for a heater in your house is typically placed near the floor. This is because hot air rises and cooler air descends. If the vent were near the ceiling, the room would still be cold near the floor because the hot air would remain near the ceiling. So hot air must be less dense than cold air. For a similar mass, it occupies a larger volume than cooler air.
How can you predict the volume of a gas sample?
To answer this question, you will explore
Predicting Gas Volume
Charles’s Law
Predicting Gas Volume
EXPLORING THE TOPIC
Predicting Gas Volume
A piston is a movable part that traps a sample of gas within a cylinder. It can move up and down if the volume of the gas changes. The illustration shows how the volume of nitrogen gas changes as the temperature changes. At first, the volume of the gas is 600 mL at a temperature of 300 K. When the cylinder is cooled, the gas contracts and the piston moves down. Then when the cylinder is heated, the gas expands and the piston moves up.
HISTORY CONNECTION
HISTORY
CONNECTION
Hydrogen gas is less dense than air. Jacques Charles took advantage of this when he invented the hydrogen balloon. In August 1783, terrified villagers destroyed the balloon when it landed outside of Paris. Charles built another hydrogen balloon, and he and a friend made the first piloted flight that December.

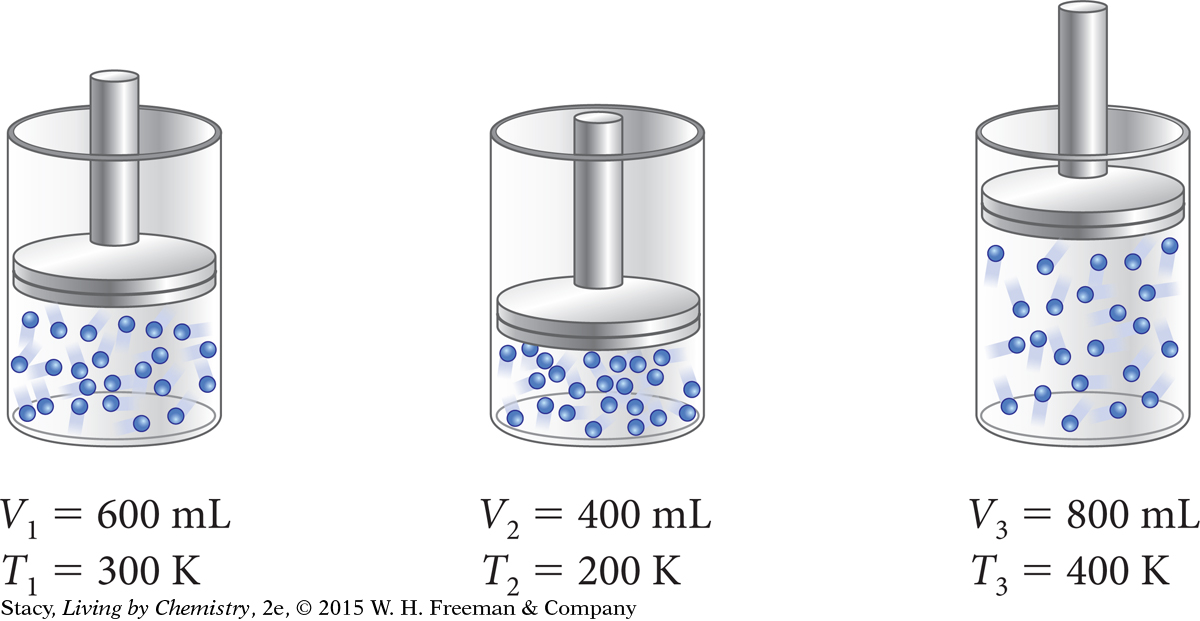
Subscripts are used to indicate corresponding volumes and temperatures for the same gas under different conditions. For example, V1 is the volume at the first temperature, T1. Notice that if you determine the ratio of the volume of the gas to the temperature of the gas, the ratio is the same at all three of these temperatures.
In fact, if nothing else changes, the ratio of volume to temperature for this gas will be 2.0 mL/K at any temperature. The relationship between the volume and temperature for this sample of gas is proportional. So the volume and temperature for this gas are related by the proportionality constant, k = V/T, or 2.0 mL/K. With this proportionality constant, you can determine the volume of this gas at any temperature. [For review of this math topic, see MATH Spotlight: Ratios and Proportions on page A-11.]
HISTORY CONNECTION
HISTORY
CONNECTION
The French Montgolfier brothers invented the first hot air balloon capable of carrying passengers. They demonstrated their creation in June 1783. The brothers thought that they had discovered a new gas that was lighter than air. In fact, the gas in their balloon was just air. It was less dense than the surrounding air because it was heated.

283
CHANGING THE AMOUNT OF GAS
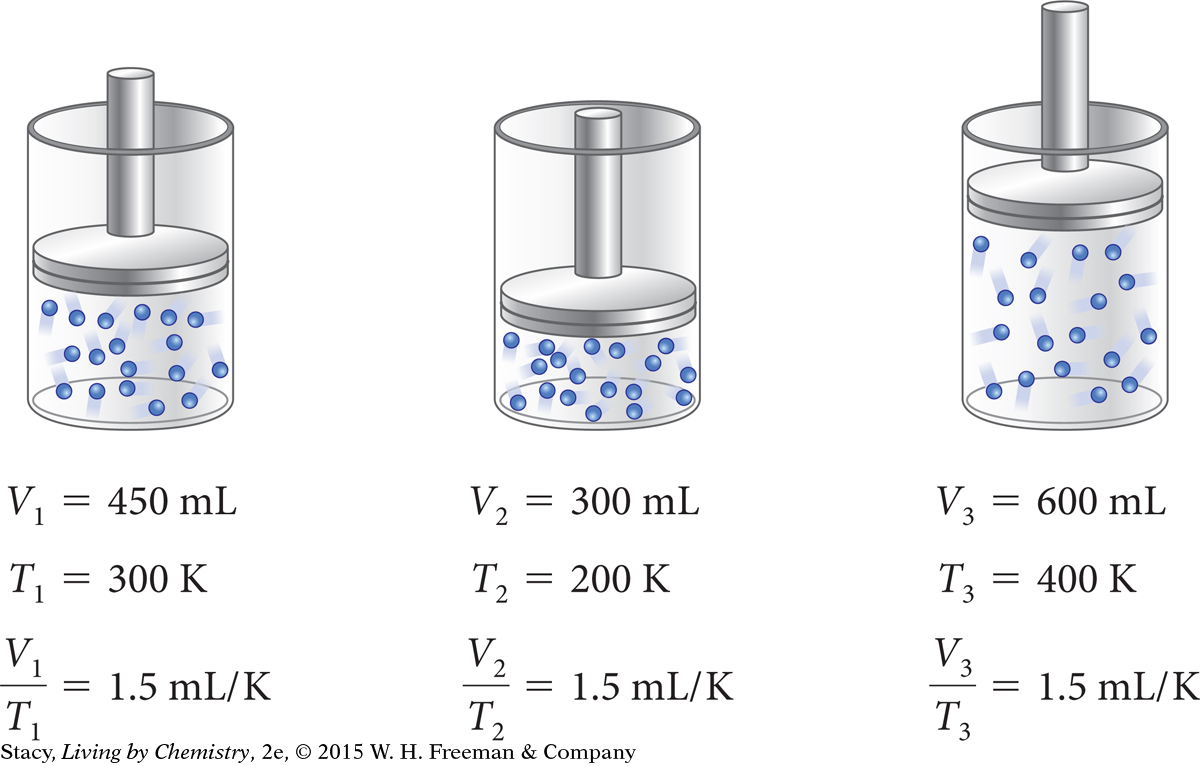
Suppose you start with a different amount of gas in the same cylinder. For this new sample of gas, the proportionality constant, k = V/T, is 1.5 mL/K.
Important to Know
Each gas sample has a unique value for the proportionality constant, k = V/T, depending on the amount of gas in the sample.
Again, you can use k = V/T for this gas sample to calculate the volume at any temperature.
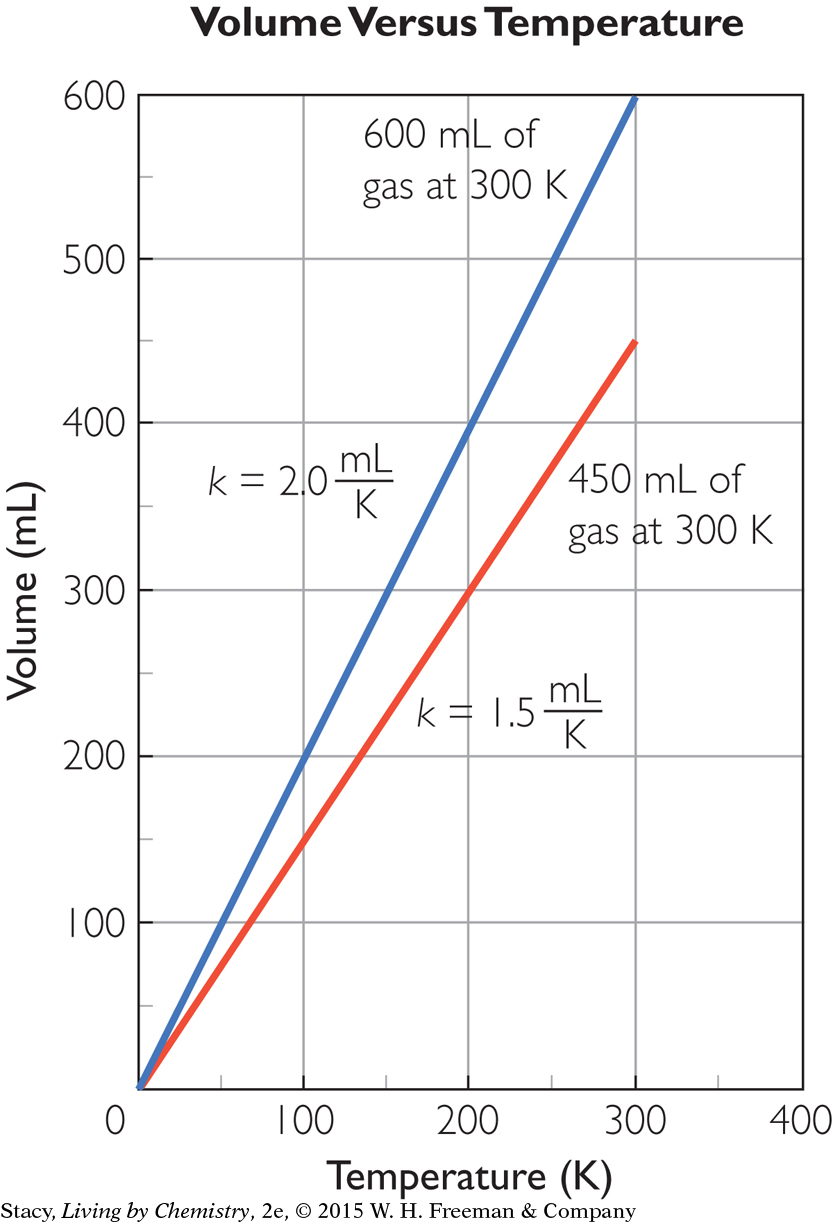
If you graph volume versus temperature for the two gas samples described previously, the result will be two different lines with different slopes.
Charles’s Law
Charles’s Law
284
Jacques Charles was a French inventor, scientist, mathematician, and very active balloonist. Because of his interest in hot air balloons, he gave a lot of thought to the relationship between the volume and temperature of a gas. In 1787, he described the proportionality of gas volume and temperature mathematically. This description is now known as Charles’s law.
Charles’s Law
If pressure and the number of particles of a gas stay the same, then volume is proportional to the Kelvin temperature.
Example 1
Calculate Gas Volume
Imagine that a cylinder with a piston contains 10 mL of air at 295 K. What will the volume of gas in the cylinder be at 550 K?
Solution
Calculate the volume by using Charles’s law or by drawing a graph. You can predict that the volume will increase when the temperature increases. So the answer should be greater than 10 mL.
Using the Formula
| Find the value of k. |
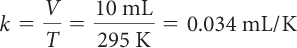
|
| Solve for the volume at 550 K. | V = kT = 0.034 mL/K · 550 K = 18.5 mL |
Graphical Analysis
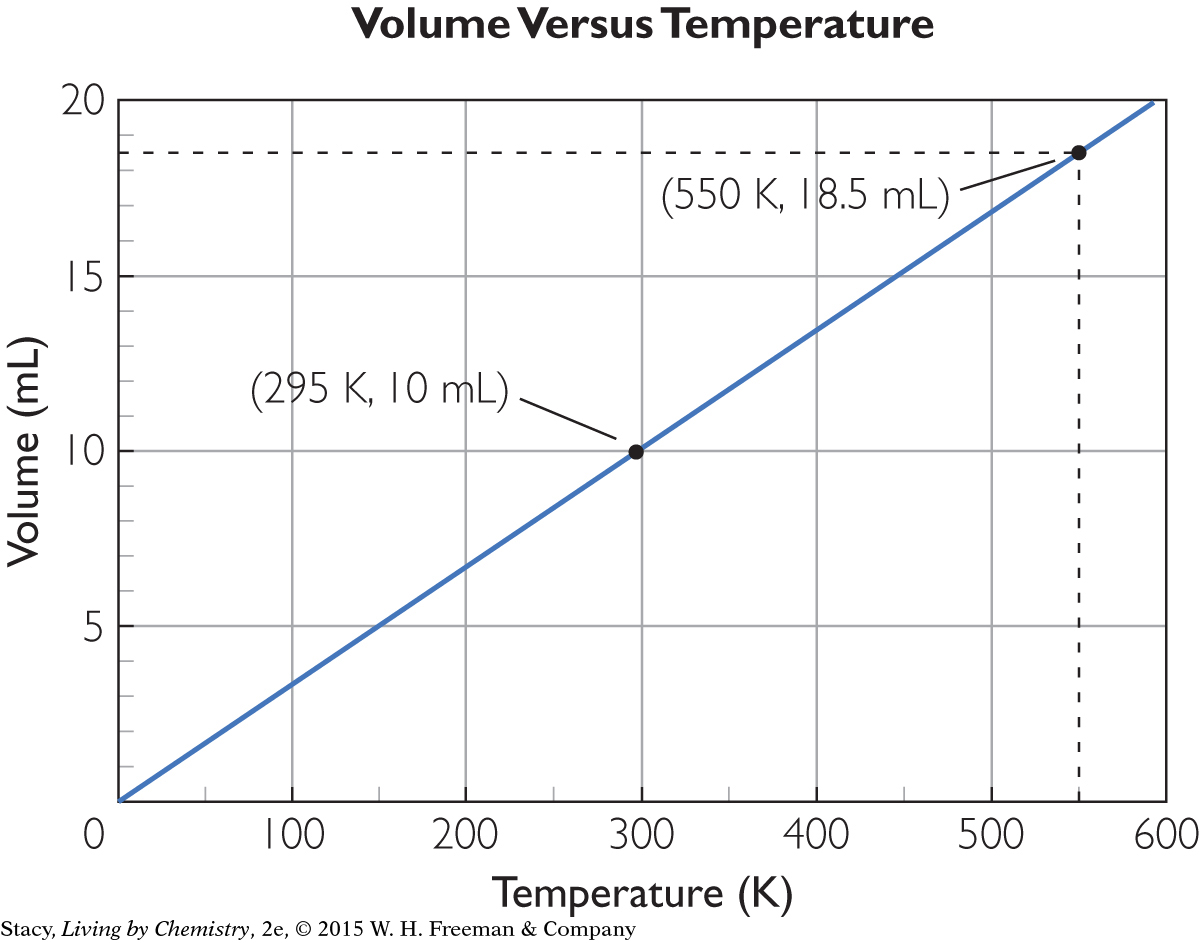
First, plot the data point for the initial conditions. Draw a line through this point and the origin, since at 0 K the y-value is theoretically 0 mL. Use the line to find the volume when the x-value is 550 K. Both methods give the same answer. At 550 K, the volume is 18.5 mL.
HISTORY CONNECTION
HISTORY
CONNECTION
On May 6, 1937, the airship Hindenburg caught fire as it landed at an airfield in New Jersey. The Hindenburg was kept afloat with massive chambers filled with hydrogen, which is less dense than air but is also highly flammable. Thirty-six people died when a fire on board spread rapidly.

285
Example 2
Calculate Gas Temperature
A balloon contains 500 mL of helium at 20 °C. You want to increase the volume to 550 mL. What temperature in °C will give you a volume of 550 mL for this sample of gas?
Solution
You can predict that the temperature will be higher for a larger volume.
| Convert the temperature to kelvins. |
K = C + 273 = 20 + 273 = 293 K |
| Determine the proportionality constant, k. |
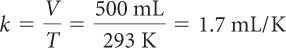
|
| Rearrange the equation in terms of T and solve for temperature at 550 mL. |
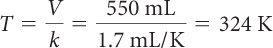
|
| Convert back to degrees Celsius. | C = K – 273 = 324 – 273 = 51 °C |
A temperature of 51 °C is needed to give a volume of 550 mL. The temperature is higher for a larger volume, as predicted.
Important to Know
To solve problems using Charles’s law, temperature values must first be converted to the Kelvin scale.
[For review of this topic, see MATH Spotlight: Solving Equations.]
LESSON SUMMARY
LESSON SUMMARY
How can you predict the volume of a gas sample?
KEY TERM
Charles’s law
The volume and temperature of a sample of gas are proportional to each other if nothing else changes (such as pressure or the amount of gas). This relationship between the volume and temperature of a gas in kelvins is described by the equation V = kT and is known as Charles’s law. The proportionality constant, k, is different for each different amount of gas.
286
Exercises
Reading Questions
Explain how to determine the proportionality constant, k, for a sample of gas.
Explain how to determine the volume of a gas at a certain temperature using the proportionality constant, k.
Reason and Apply
A gas sample in a cylinder has a volume of 620 mL at 293 K. If you allow the piston to move while you heat the gas to 325 K, what will the volume of the gas be? Check your answer by drawing a graph.
A gas sample in a cylinder has a volume of 980 mL at a temperature of 27 °C. If you allow the piston to move while you heat the gas to 325 K, what will the volume of the gas be at 325 K?
A gas sample in a cylinder with a piston has a volume of 330 mL at 280 K. What temperature will you need to heat it to in order to change the volume to 220 mL?
A 2.0 L gas sample at 20 °C must be cooled to what temperature for the volume to change to 1.0 L? Show at least two different ways to solve this problem.
Imagine that you have a huge helium balloon for a parade. Around noon, it is 27 °C when you fill the balloon with helium gas to a volume of 25,000 L. Later in the day, the temperature drops to 15 °C.
What is the proportionality constant, k = V/T, at the beginning of the day?
Calculate the volume of the balloon when the temperature has dropped to 22 °C.
What will the proportionality constant, k = V/T, be at the end of the day when the temperature is 15 °C? Explain your answer.
Would this cup make a good rain gauge? Explain your thinking.
 Petr Jilek/Shutterstock
Petr Jilek/Shutterstock