LESSON 56: It’s Sublime
295
THINK ABOUT IT
Earth is unique in that it is the only planet in the solar system with so much liquid water on its surface. The weather and the life on Earth depend on the movement of moisture around the planet. The phase changes of water are mostly responsible for this movement. When water changes phase from liquid to gas, the airborne water molecules spread out in the atmosphere and the density changes dramatically.
How do the densities of a solid and a gas compare?
To answer this question, you will explore
Density of Gases
The Molecular View
Comparing Density and Phase of Water
Density of Gases
EXPLORING THE TOPIC
Density of Gases
An airplane glides easily through the air at around 500 miles per hour. The density of air must be very low for objects to pass so easily through it. Determining the density of a gas is not as straightforward as determining the densities of liquids and solids. This is because it is not easy to measure the mass of a gas. It is hard to put a gas on a balance, and many gases, like helium, rise up in the air around them.
One approach is to measure the mass of a substance while it is a liquid or a solid, and then turn it into a gas. Provided you don’t let any escape, the substance will have the same mass as a gas as it did as a liquid or a solid. You can then measure the volume of the gas to determine its density.
SUBLIMATION OF DRY ICE

One substance that you can use to study phase changes is carbon dioxide, CO2. Carbon dioxide turns directly from a solid to a gas at the extremely low temperature of −78 °C (−108 °F). This means the temperature in your classroom is warm enough to turn solid carbon dioxide into a gas.
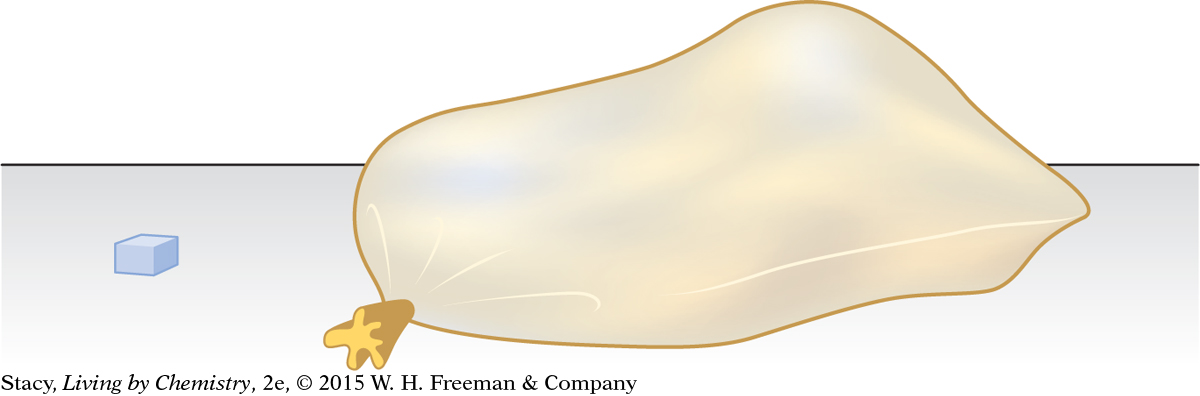
When a solid changes directly into a gas without forming a liquid, the phase change is called sublimation. In class, you gathered data on the density of CO2(g) by finding the mass of a chunk of CO2(s) and then placing it into a plastic bag and allowing it to sublime. The volume of the gas is huge compared to the volume of the same mass of solid.
296
The density of the carbon dioxide changes dramatically as the phase changes. CO2(s) has a density of 1.56 g/mL. In contrast, CO2(g) has a very low density of 0.0019 g/mL. The volume of the gas is nearly 800 times as large as the volume of the solid!
Big Idea
Big Idea
The densities of gases are extremely low compared to the densities of liquids and solids.
The Molecular View
The Molecular View
Why did the bag puff up so much as the CO2(s) sublimed? Because you cannot see gases, you must depend on hypotheses about how the molecules in a gas behave. Consider four possible hypotheses and the corresponding models for the sublimation of solid carbon dioxide.
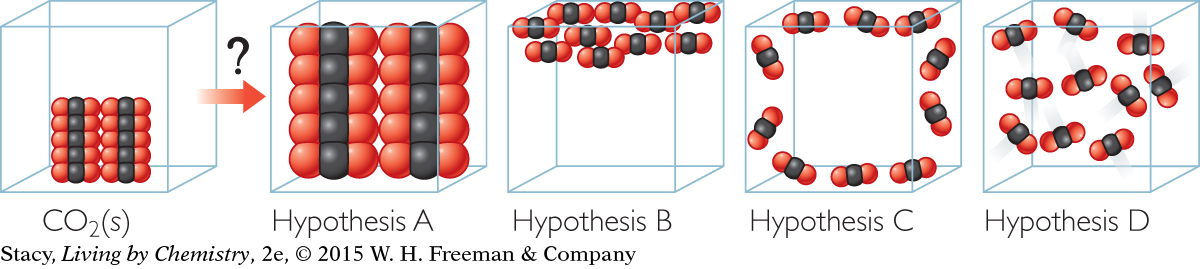
Hypothesis A: The CO2 molecules become larger in the gas phase compared with the solid phase.
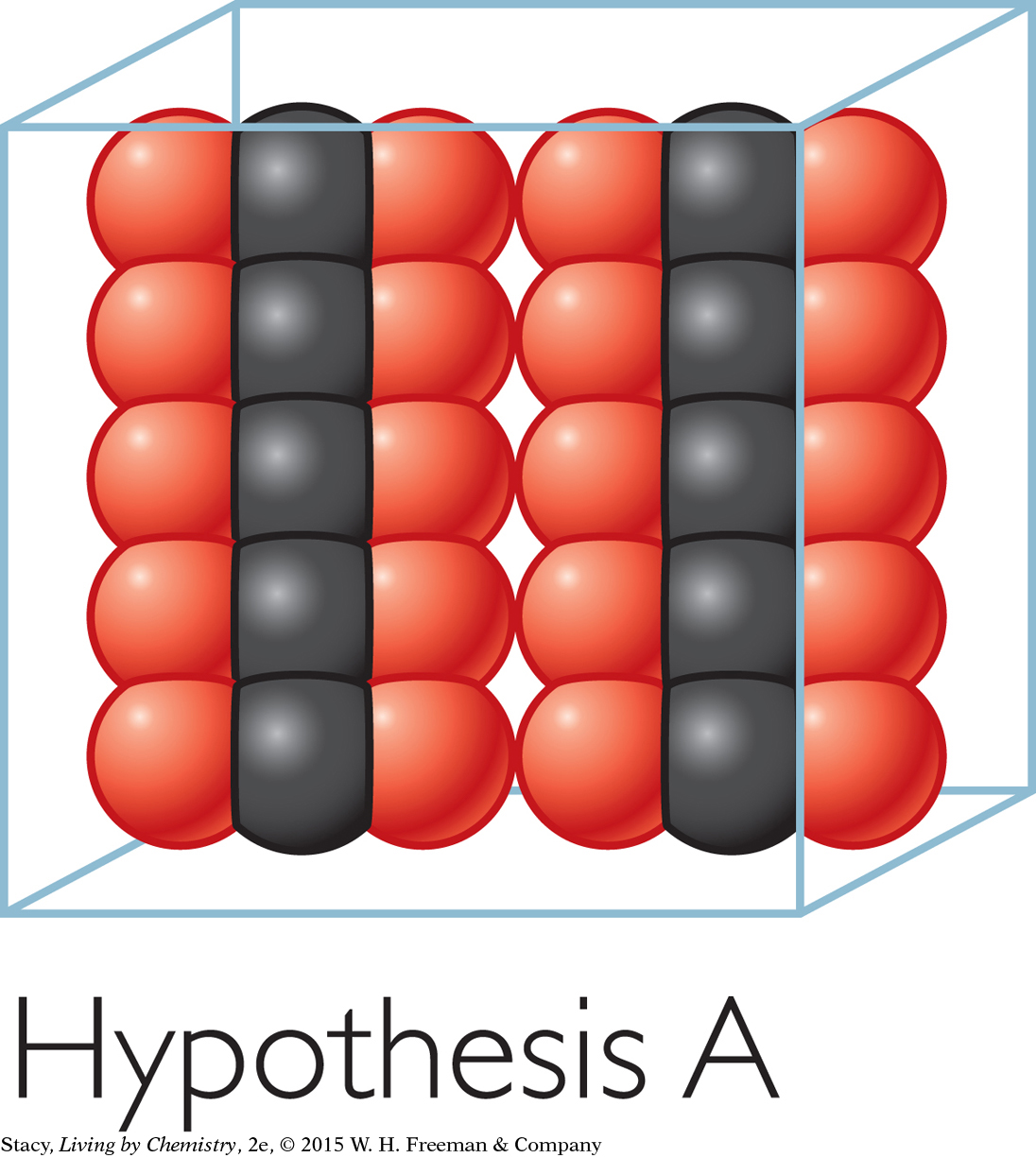
Evidence against Hypothesis A: You can move freely through a gas, so the molecules must not take up much space. The mass stays the same, so it is difficult to imagine how the molecules would get bigger. The evidence doesn’t support Hypothesis A.
Hypothesis B: The CO2 molecules rise to the top of the container.
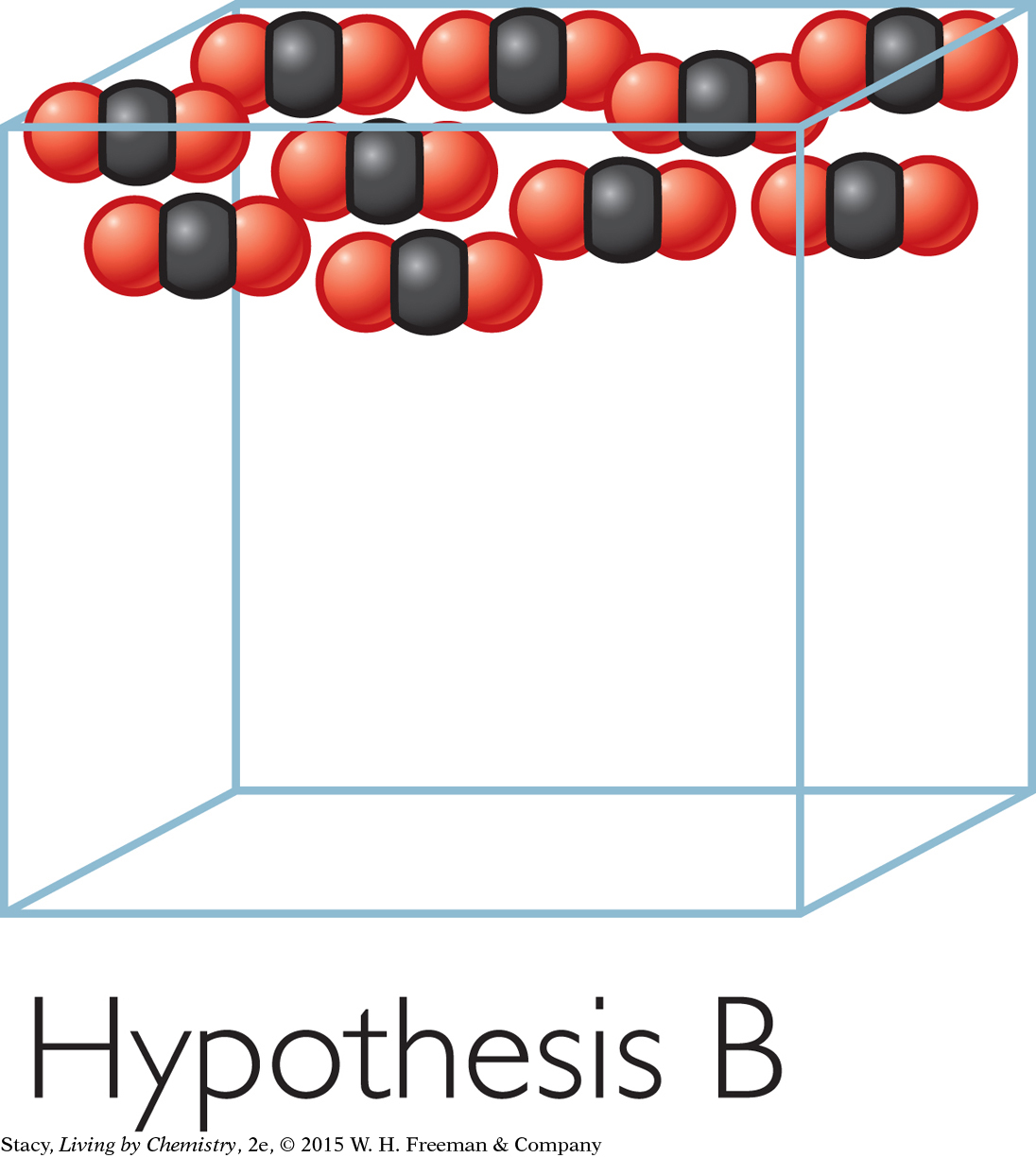
Evidence against Hypothesis B: The bag filled with CO2(g) was pushed out in all directions. This indicates that the molecules are spread throughout, not just at the top. For example, the air in a room is everywhere, not just near the ceiling. The evidence doesn’t support Hypothesis B.
Hypothesis C: The molecules go to the outer edges of the bag, pushing the sides out.
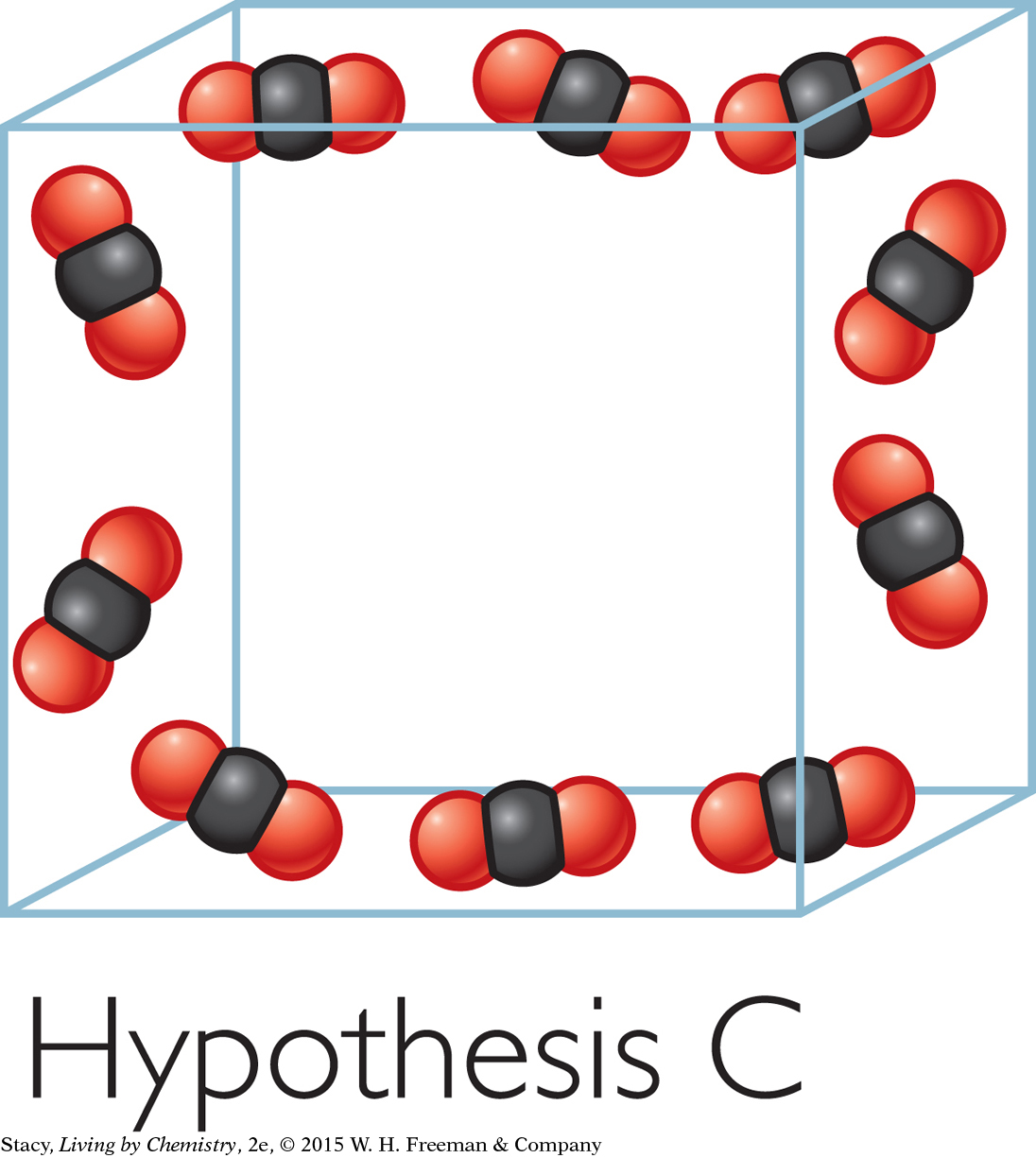
Evidence against Hypothesis C: If gases went to the outer edges of their containers, you would walk into a room and find all the air on the walls and none in the middle. Since you can breathe everywhere in the room, this indicates that gas molecules are spread throughout. The evidence doesn’t support Hypothesis C.
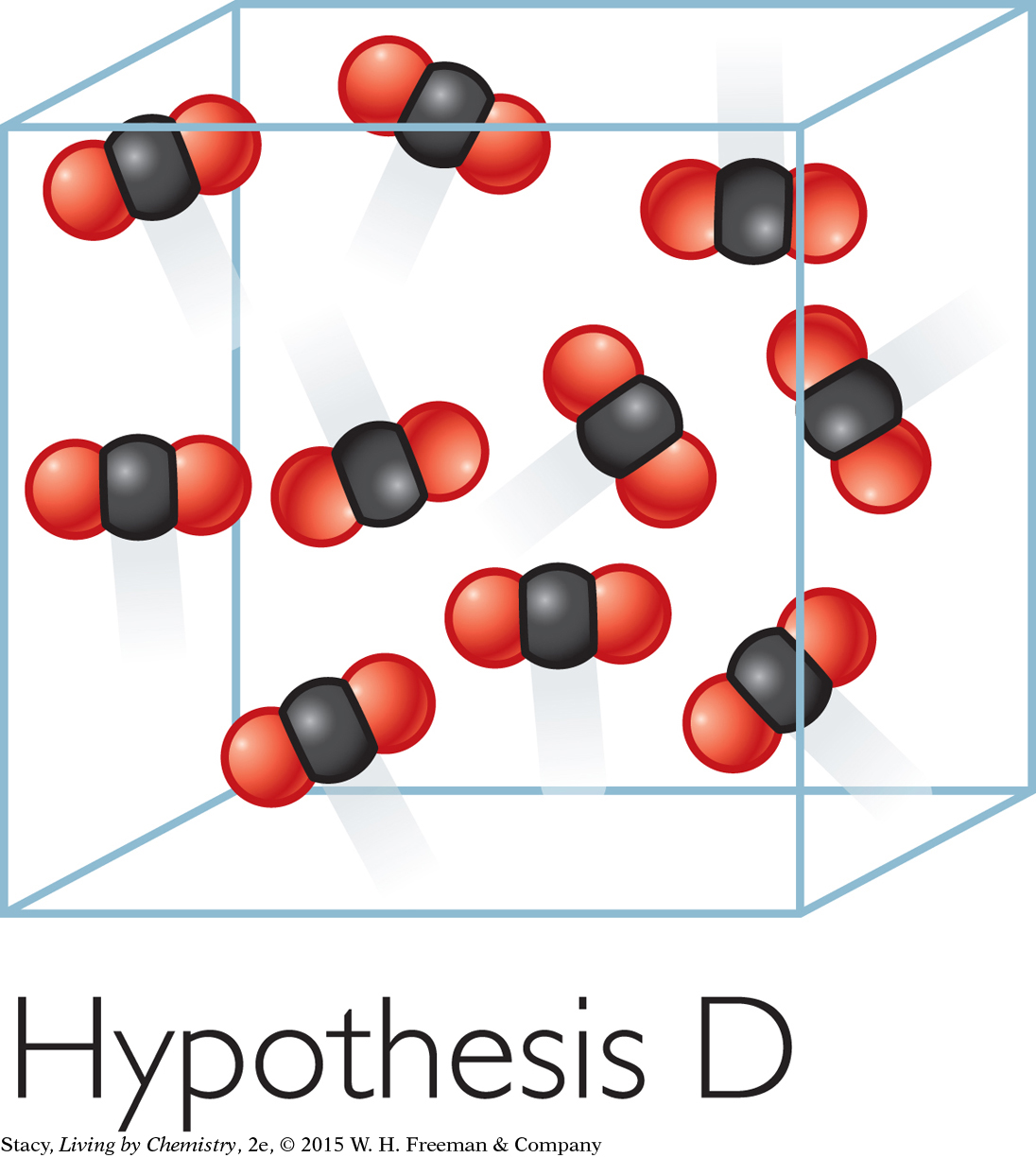
Hypothesis D: The CO2 molecules spread apart from one another and bounce around inside the bag.
Evidence supporting Hypothesis D: This model is consistent with the large volume and the low density of the gas. This model is also consistent with the bag staying puffed out in all directions and offering resistance when you push on it. This evidence supports Hypothesis D.
Model D can still be improved upon. It shows ten molecules in a small cubic space. In actuality, the gas molecules are much tinier, much farther apart, much more numerous, and moving very fast. When a gas is trapped in a bag, the moving molecules bang into the bag and push it out. If you were to remove the bag, the molecules would quickly spread throughout the room. This is what happens to water when it evaporates, or changes into a vapor, and spreads out in the atmosphere.
297
Big Idea
Big Idea
A sample of gas expands to fill whatever space it is in.
Comparing Density and Phase of Water
Comparing Density and Phase of Water

The table shows the density for some different forms of water. Water has three phases: solid, liquid, and gas. But these phases can take a few different forms. For example, snow and ice both represent solid forms of water.
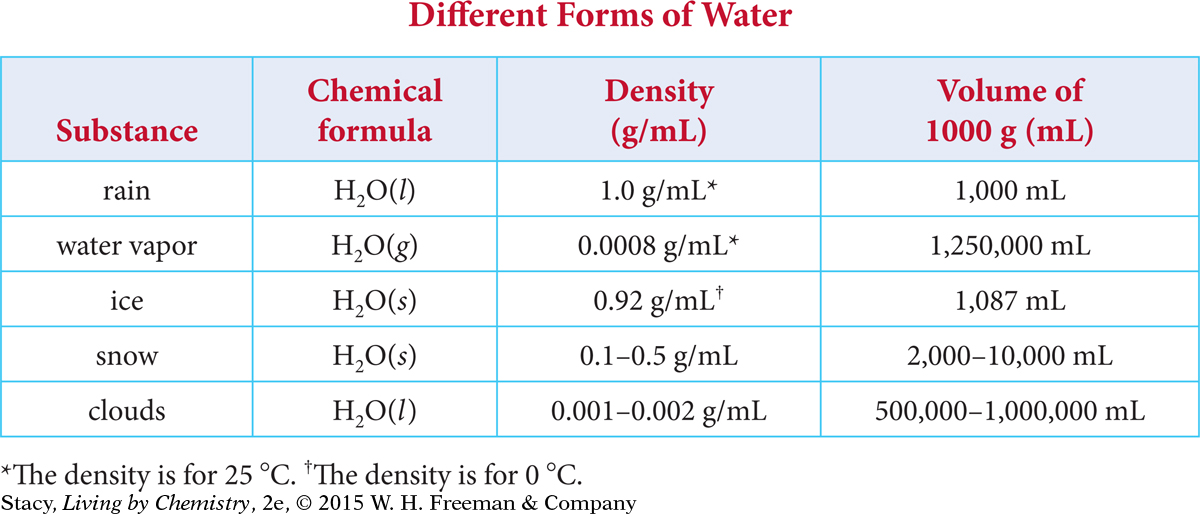
Notice what happens to the volume of 1000 grams of water as it goes from liquid to water vapor. The volume increases more than a thousand-fold when evaporation occurs.
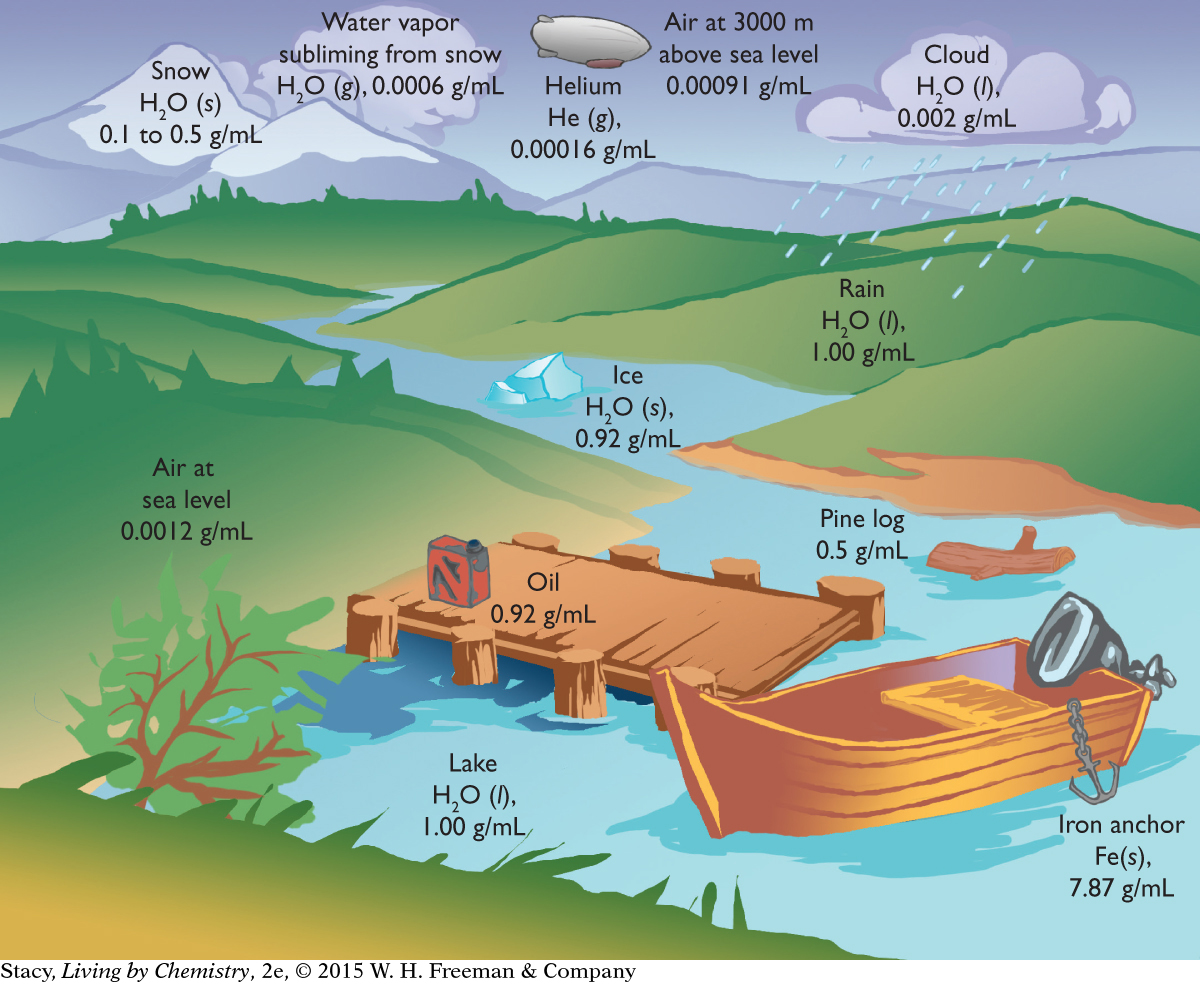
The density landscape illustration shows the relationship between the location of matter and its density.
ASTRONOMY CONNECTION
ASTRONOMY
CONNECTION
The lowest temperature ever recorded on Earth was −129 °F in 1983 in Antarctica. At this temperature, CO2(g) condenses to form solid dry ice. The southern polar ice cap on Mars contains dry ice because the temperature there is below the sublimation temperature of CO2.

298
Substances that are less dense float in substances that are denser. For example, ice floats in liquid water. When the temperature of a mass of air increases, the air expands, resulting in a lower density. This change in density causes the warmer air to rise above cooler air. These changes are related to the movement of matter on the planet and are essential to causing different weather.
LESSON SUMMARY
LESSON SUMMARY
How do the densities of a solid and a gas compare?
KEY TERMS
sublimation
evaporation
Gases form when solids sublime and liquids evaporate. During both sublimation and evaporation, molecules spread far apart from each other. This increases the volume of the substance by about 1000 times. It also decreases the density of the substance dramatically. Solids are generally denser than liquids. Gases are much less dense than both solids and liquids. Gases expand to fill whatever container they are in.
Exercises
Reading Questions
How does the density of a gas compare with the density of a solid?
Draw a molecular view of carbon dioxide gas.
Reason and Apply
In your own words, define sublimation and evaporation.
Describe three ways to show that gases exist.
When water freezes, the water molecules move apart very slightly. What evidence can you provide to support this claim?
Draw a molecular view for water vapor, liquid water, and ice.
What is the volume of 6.4 g of CO2(s)? (density = 1.56 g/mL)
What is the mass of 3.5 L of CO2(g)? (density = 0.0019 g/mL)
How many grams of CO2(s) would you need to fill a 6.5 L bag with CO2(g)?
A person has 15 g of dry ice and wants to completely fill a bag that has a volume of 8 L with carbon dioxide gas. Is this enough dry ice to fill the bag with CO2(g)? Explain.