LESSON 57: Air Force
299
THINK ABOUT IT
Earth is surrounded by the atmosphere, a layer of air nearly 80 miles thick. The atmosphere is made up predominantly of colorless, odorless gases, which you can move through. The air molecules are tiny, and they are spaced far apart, so most of the time it feels as if nothing is there. However, the weight of all that air above you exerts a great deal of pressure on you.
What evidence do we have that gases exert pressure?
To answer this question, you will explore
Evidence of Air Pressure
Explanation for Air Pressure
The Atmosphere
Evidence of Air Pressure
EXPLORING THE TOPIC
Evidence of Air Pressure
HISTORY CONNECTION
HISTORY
CONNECTION
Atmospheric pressure was demonstrated in 1654 when Otto von Guericke, of Magdeburg, Germany, used a vacuum pump to remove almost all of the air from the space between two copper hemispheres that were 1.6 ft in diameter. The air pressure holding the two halves together was so strong that two teams of horses could not pull them apart. However, when air was let back in, the hemispheres fell apart easily.

When you fill a car’s tires with air, the rubber is stretched quite tightly. The pressure exerted by the air in the tires is high enough to push the entire car up off the ground. Indeed, the air pressure is holding up more than 2000 pounds. How can air be so powerful?
The ability of car tires to remain inflated while holding up so much weight provides evidence that air is pushing on the inside of the tire in all directions. This “pushing” property of a gas is called pressure. Pressure is defined as a force over a specific surface area. A gas exerts pressure on all surfaces it comes in contact with.
There are many ways to demonstrate that gases exert pressure on the things around them. Here are two examples.
SUBMERGED PAPER

When a plastic cup is turned upside down and submerged in water, the paper inside the cup stays dry. There is air trapped in the cup that pushes out in all directions. The air pushes on the water with enough pressure to keep the water out of the cup.
BALLOON IN A BOTTLE
Suppose you try to blow up a balloon inside a bottle. Even with a great deal of effort, the balloon will inflate only a tiny bit. The balloon does not inflate because there is air inside the bottle. Even though it looks like “nothing is there,” the bottle is full of air, and when you try to inflate the balloon, you are pushing on this air, which takes a lot of work!
300
PHYSICS CONNECTION
PHYSICS
CONNECTION
A gas can even push against another gas. In fact, gas with a high pressure pushes a space shuttle into the air.


Explanation for Air Pressure
Explanation for Air Pressure
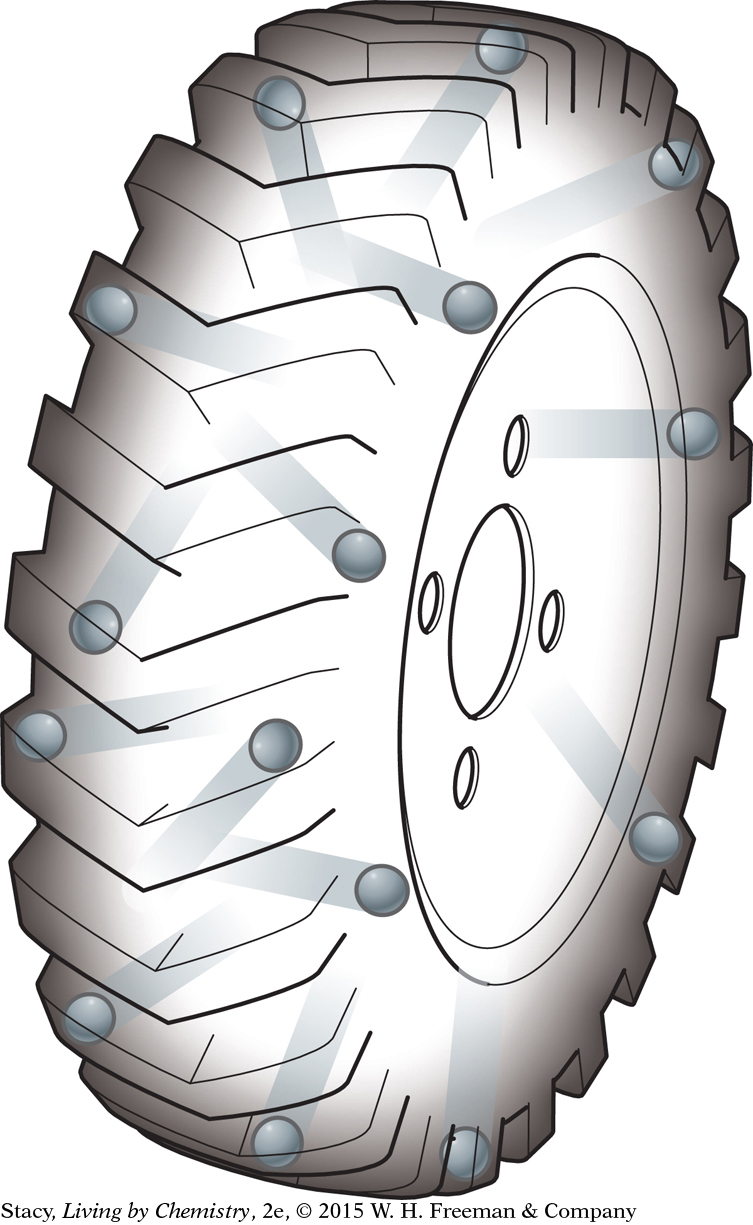
Collisions of gas molecules with surrounding objects are what we experience as air pressure. There are huge numbers of molecules in an automobile tire, many more than are shown in the illustration. The tiny push from each one adds up to a lot, enough to hold a car up.
The pressure of the air inside your tires is expressed in pounds per square inch, or lb/in2. This unit is sometimes written as psi, for pounds per square inch. That is, each square inch of surface area on the tire experiences a certain number of pounds of force from the air inside. A tire on a racing bicycle might be inflated to a pressure of 100 lb/in2.
Example
Air Pressure in Car Tires
Suppose a car weighs 2000 pounds. Each of its four tires touches the road over an area that is about a 4-by-4-inch square. What air pressure do you need in each tire to hold up the car?
Solution
Each of the four tires needs to push up ¼ of the 2000 pounds, or 500 pounds. A 4-by-4-inch square has an area of 16 in2, so each tire is in contact with a 16 in2 area of the ground. Pressure is force per unit area.
Pressure = force/area
= 500 lb/16 in2
= 31 lb/in2
The pressure in each tire needs to be at least 31 lb/in2.
The Atmosphere
The Atmosphere
301
Earth’s atmosphere is a mixture of gases, mostly nitrogen, with some oxygen, carbon dioxide, water vapor, and argon. This mixture of gases is what we call air. The density of Earth’s atmosphere changes as you travel up in altitude—the air becomes thinner at higher elevations. This concept will be covered more extensively in later lessons.
Big Idea
Big Idea
The atmosphere is a mixture of gases, including gaseous water.
CONSUMER CONNECTION
CONSUMER
CONNECTION
Suction cups are misnamed. There is nothing pulling inside the suction cup. The atmosphere is pushing on the outside, and there are fewer molecules on the inside to push back.

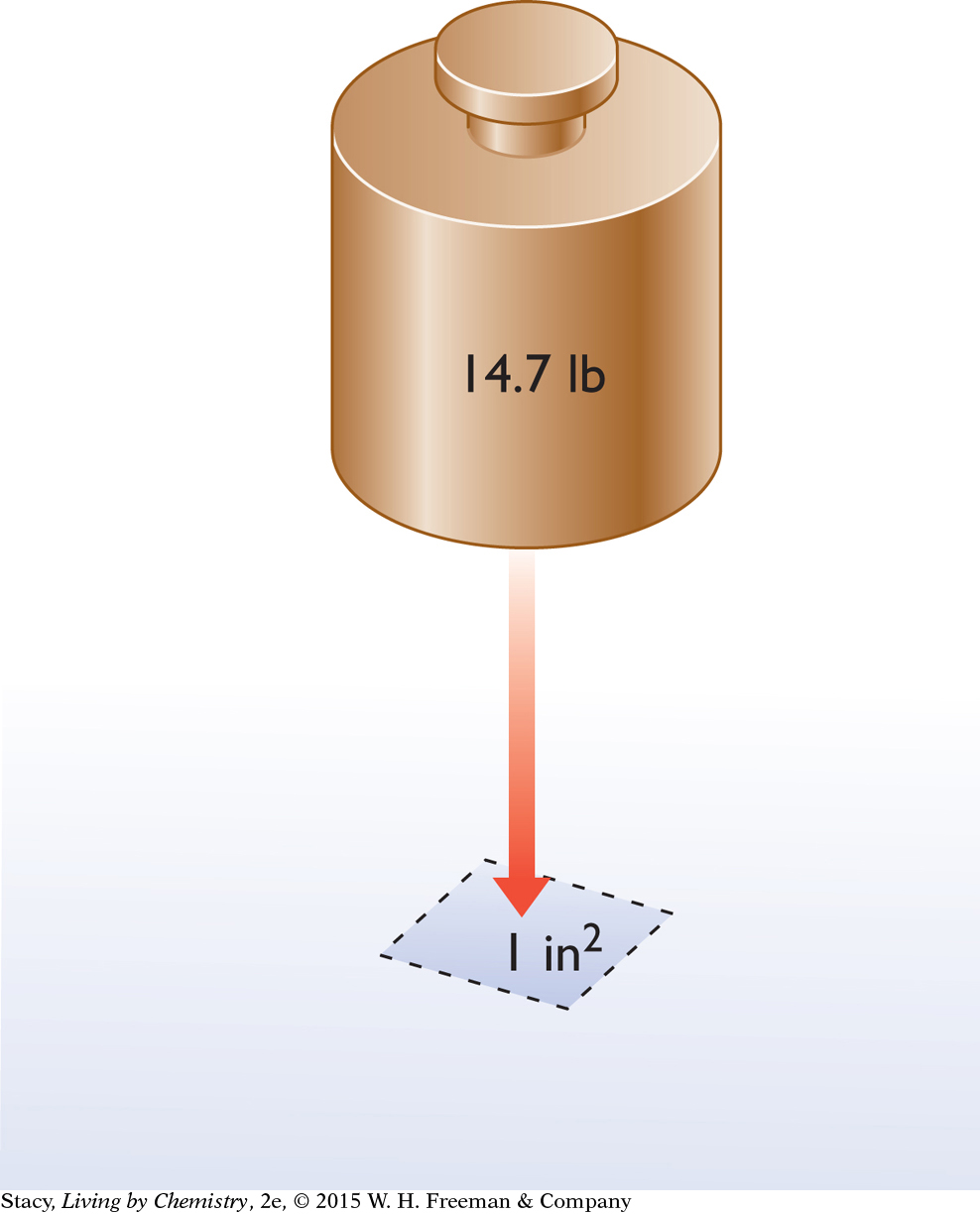
Although we barely notice it, the air around us exerts pressure on us all the time. Atmospheric pressure is air pressure that is always present on Earth as a result of air molecules colliding with objects on the planet. The pressure due to the atmosphere is the equivalent of a 14.7-pound weight pushing on every square inch of surface. We say that the pressure due to the atmosphere is 14.7 pounds per square inch, or 14.7 lb/in2. This pressure is measured at sea level.
Scientists also use a unit called an atmosphere, or atm, to measure air pressure.
Atmospheric Pressure At sea level and 25 °C, there is 1 atm of pressure.
1 atm = 14.7 lb/in2
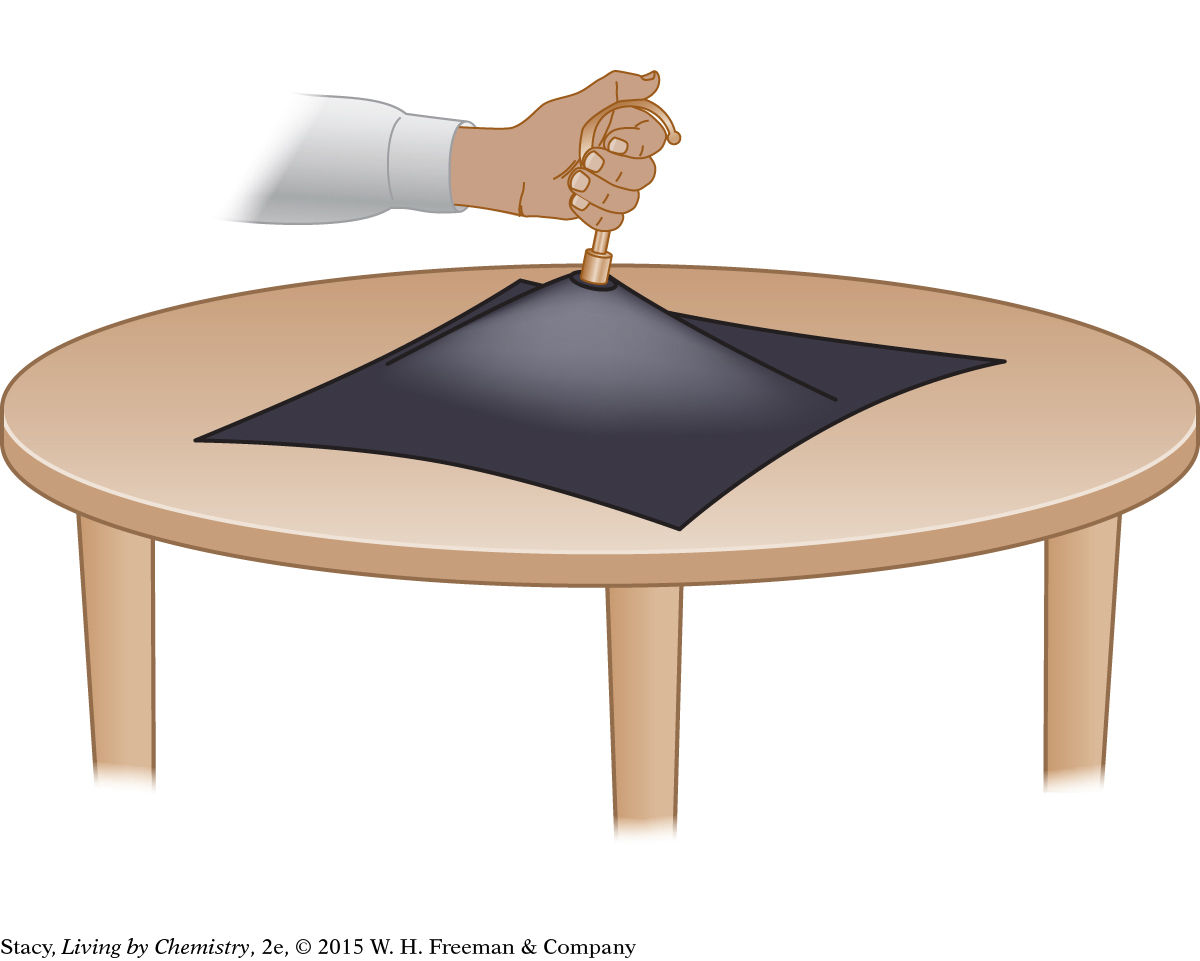
Atmospheric pressure played a role in all of the demonstrations you completed in class. The air pressure mat is particularly good at demonstrating how much pressure the air around us exerts on objects. If the mat has a surface area of 100 square inches and standard air pressure from the atmosphere is 14.7 lb/in2, the total pressure on the mat from the air is 1470 pounds! No wonder no one can lift it.
LESSON SUMMARY
LESSON SUMMARY
What evidence do we have that gases exert pressure?
KEY TERMS
pressure
atmospheric pressure
atmosphere (atm)
Earth is surrounded by an atmosphere of gases. We call this mixture of gases air. Although we cannot see it, the air around us exerts pressure. Gas molecules are constantly moving and colliding with anything they come in contact with. Air pressure is defined as the force over a surface area. This force is caused by the collisions of the gas molecules. Gas pressure is measured in pounds per square inch, lb/in2, or in atmospheres, atm. At sea level and 25 °C, the atmospheric pressure is 14.7 lb/in2, or 1 atm.
302
Exercises
Reading Questions
Give three pieces of evidence that air exerts pressure.
Explain what causes air pressure.
Reason and Apply
High up in space, there are no molecules to collide with the outside walls of a balloon. Therefore, the air pressure inside the balloon is much greater than the air pressure outside of the balloon. Describe what would happen to an inflated balloon if it were suddenly put in space.
Calculate the weight in pounds of the atmosphere pushing down on the back of your hand.
When you fly in an airplane, it is common to feel painful pressure in your ears. Explain what might be happening in terms of air pressure.
What is the minimum pressure you need in bike tires to hold up a person who weighs 150 lb? Assume that the tires touch the ground in a square that is 1.5 in by 1.5 in.
The air pressure from the atmosphere measures 0.5 atm at an altitude of 18,000 ft. How much pressure is this in pounds per square inch? Calculate the weight in pounds of the atmosphere pushing down on the back of your hand at this altitude.
Go to a library or do a Web search to find out how gases, such as helium or oxygen, are transported for industrial use. If a car runs over the tip of your foot, it doesn’t break your toes. Explain why.