Featured LAB: Boyle’s Law
Featured LAB
303
Boyle’s Law

Purpose
To observe and quantify the relationship between gas pressure and gas volume.
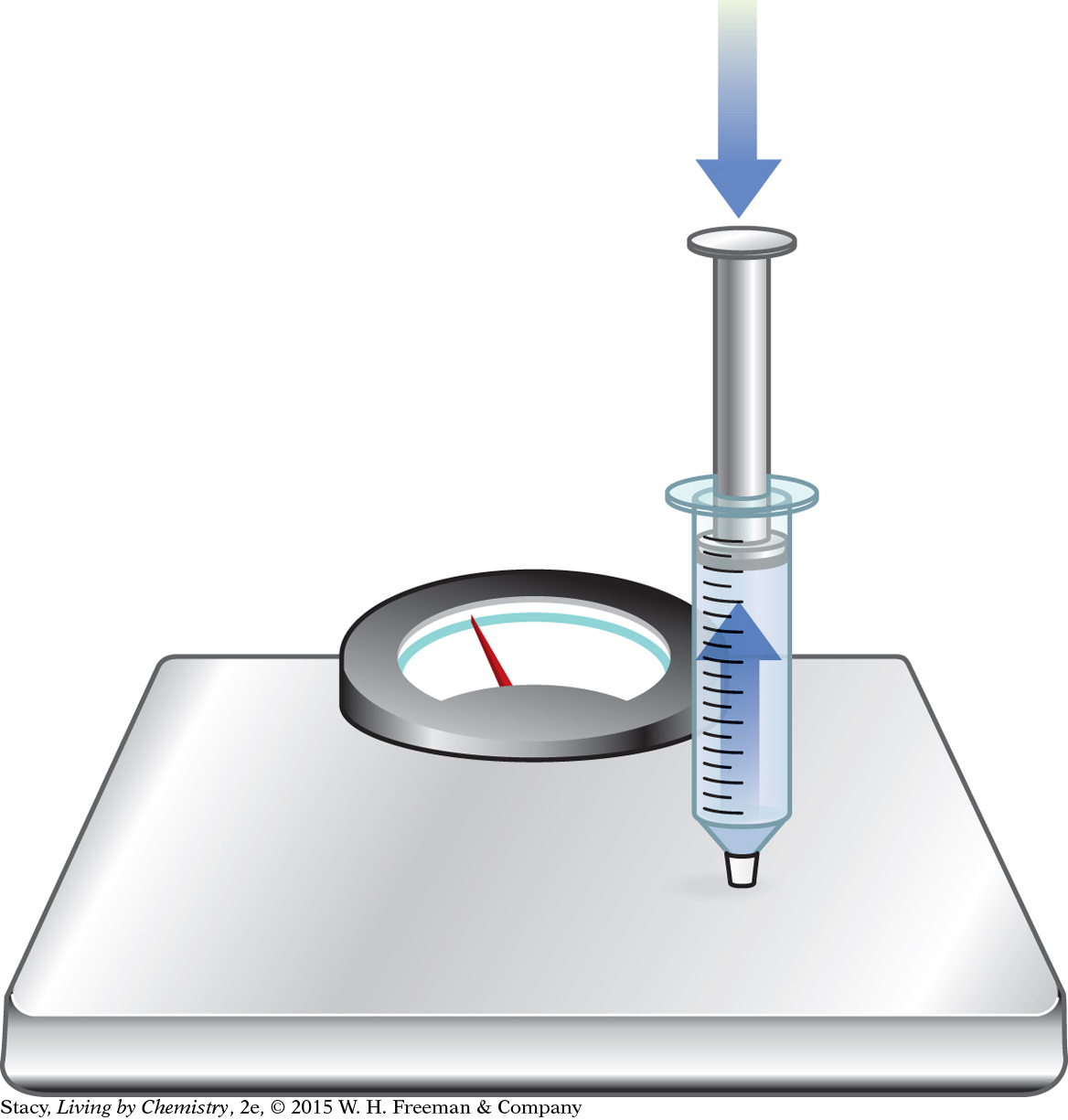
Materials
50 mL plastic syringe with cap screwed on tight
bathroom scale
Procedure
Start with the syringe at 50 mL. Make sure the cap is on tight.
Hold the syringe so that the tip is on top of a bathroom scale.
Repeat these steps for at least five different volumes.
One person depresses the plunger by a few milliliters.
A second person reads the exact volume.
A third person reads the number of pounds that is exerted on the bathroom scale.
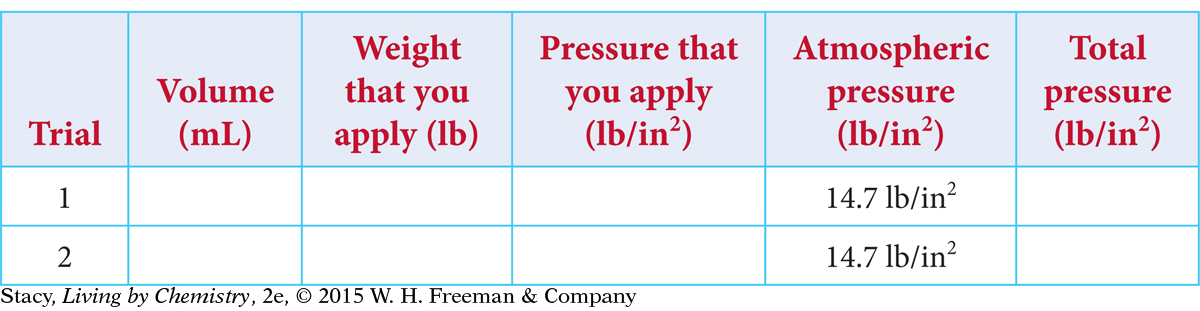
Everyone records the volume and weight data in a table like this one.
Data
!
SAFETY
Instructions
!
The cap on the tip of the syringe should always be pointed down, away from eyes. Wear safety goggles.
Analysis
Estimate the cross-sectional area inside of the syringe in square inches. Determine the pressure applied in pounds per square inch. Calculate the rest of the values needed to complete your table.
Graph the total pressure versus volume. What happens to the pressure of the gas as the volume of the gas decreases?
Making Sense Using today’s observations, explain how the pressure and volume of a gas change in relation to each other.