LESSON 60: Be the Molecule
THINK ABOUT IT
314
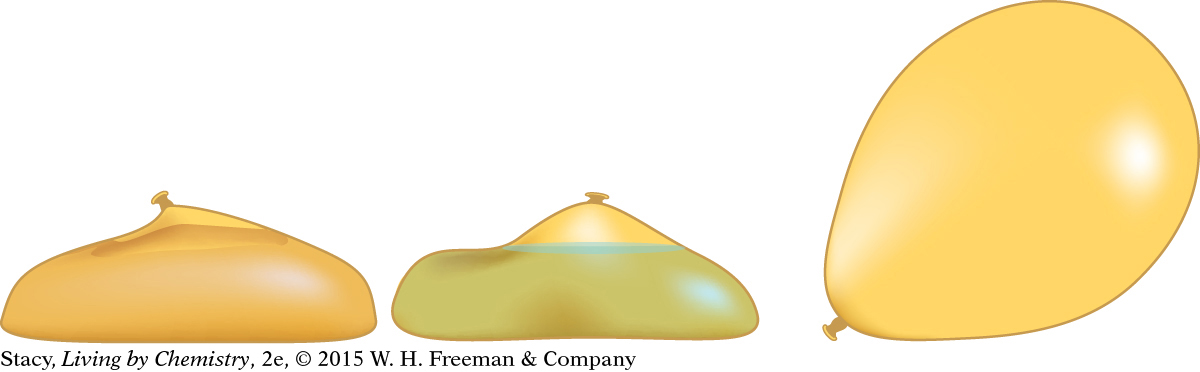
Imagine you have three balloons: one filled with sand, a second filled with water, and a third filled with air. The air balloon has the least mass but it has the largest volume. It is also the most uniform and spherical, indicating that the gas molecules are pushing out in all directions.
How do molecules cause gas pressure?
To answer this question, you will explore
Kinetic View of Pressure
Changing Gas Pressure
Kinetic View of Pressure
EXPLORING THE TOPIC
Kinetic View of Pressure
Inside the sand balloon, the atoms that make up sand are tightly packed together. Inside the water balloon, the forces between the water molecules keep them close to each other but not quite as close as the molecules in solids. Inside the air balloon, however, there is a lot of movement and activity. The gas molecules are moving rapidly and bouncing off each other and the walls of the balloon.
Big Idea
Big Idea
Gas pressure is caused by the collisions of molecules or atoms with all surfaces such as the walls of a container or your skin.
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
The diaphragm is a muscle that separates the chest from the abdomen. When the diaphragm moves up, air flows out of your nose and mouth. When the diaphragm moves down, the volume of the chest cavity increases, allowing air to flow in.
COLLISIONS CAUSE PRESSURE
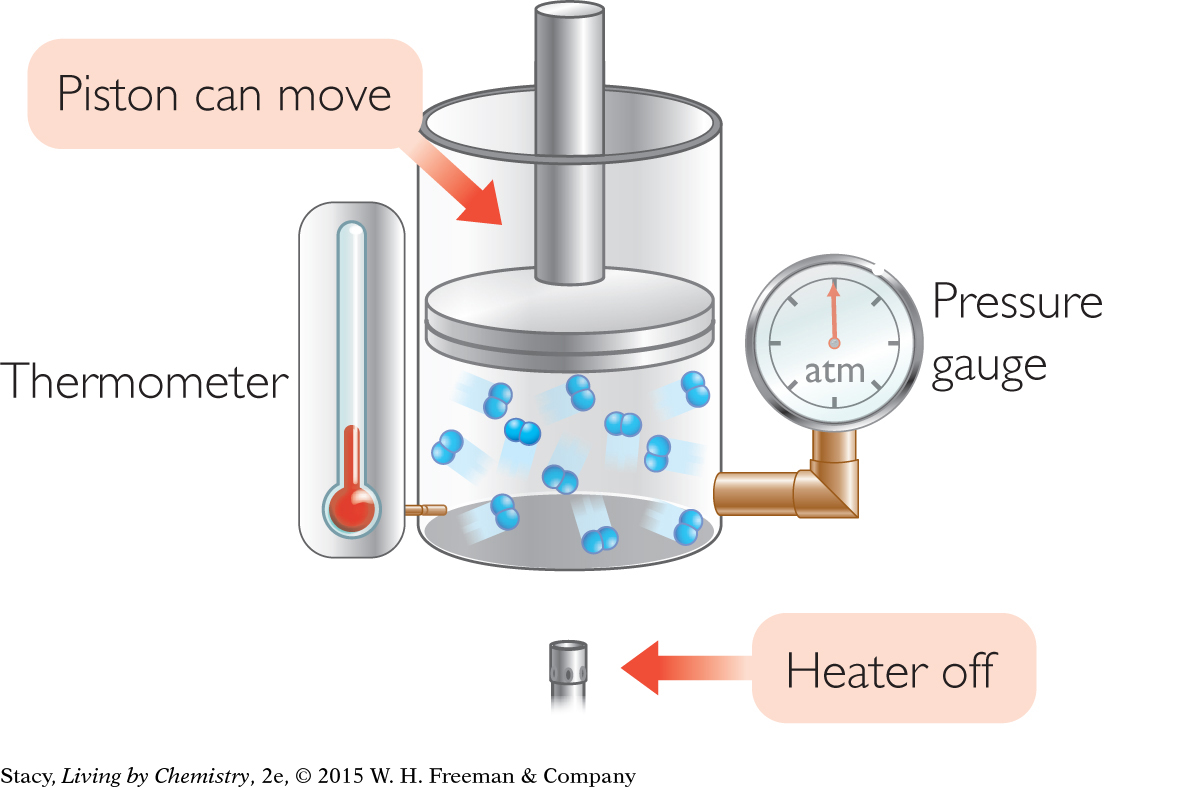
According to the kinetic theory of gases, gas molecules are in constant motion. The gas molecules in a sample move at many hundreds of miles per hour in straight line paths. Even though they are very tiny, the molecules inside the container hit each other and the walls of their container. There are so many molecules hitting the walls that the collisions add up to a measurable pressure.
Note that gas pressure is caused by molecules hitting the walls of the container only. When gas molecules collide with each other, this may change their speed or direction, but it does not affect the overall gas pressure.
315
The illustration shows a gas trapped in a container with a piston that can move up and down. The temperature and pressure of the gas inside the container are indicated on a thermometer and pressure gauge. Notice that the gas inside the container exerts enough pressure on the piston to hold it up. The piston does not fall downward, even though the gas molecules are very far apart.
Changing Gas Pressure
Changing Gas Pressure
Look again at the illustration. Suppose you keep the temperature the same but decrease the volume of the cylinder. What happens to the gas molecules inside the container? There is less space for the same number of molecules. The pressure increases. If you keep the volume the same but heat the gas inside the container, the gas molecules move faster. The pressure increases again. The drawings here illustrate these two changes.
CHANGING VOLUME
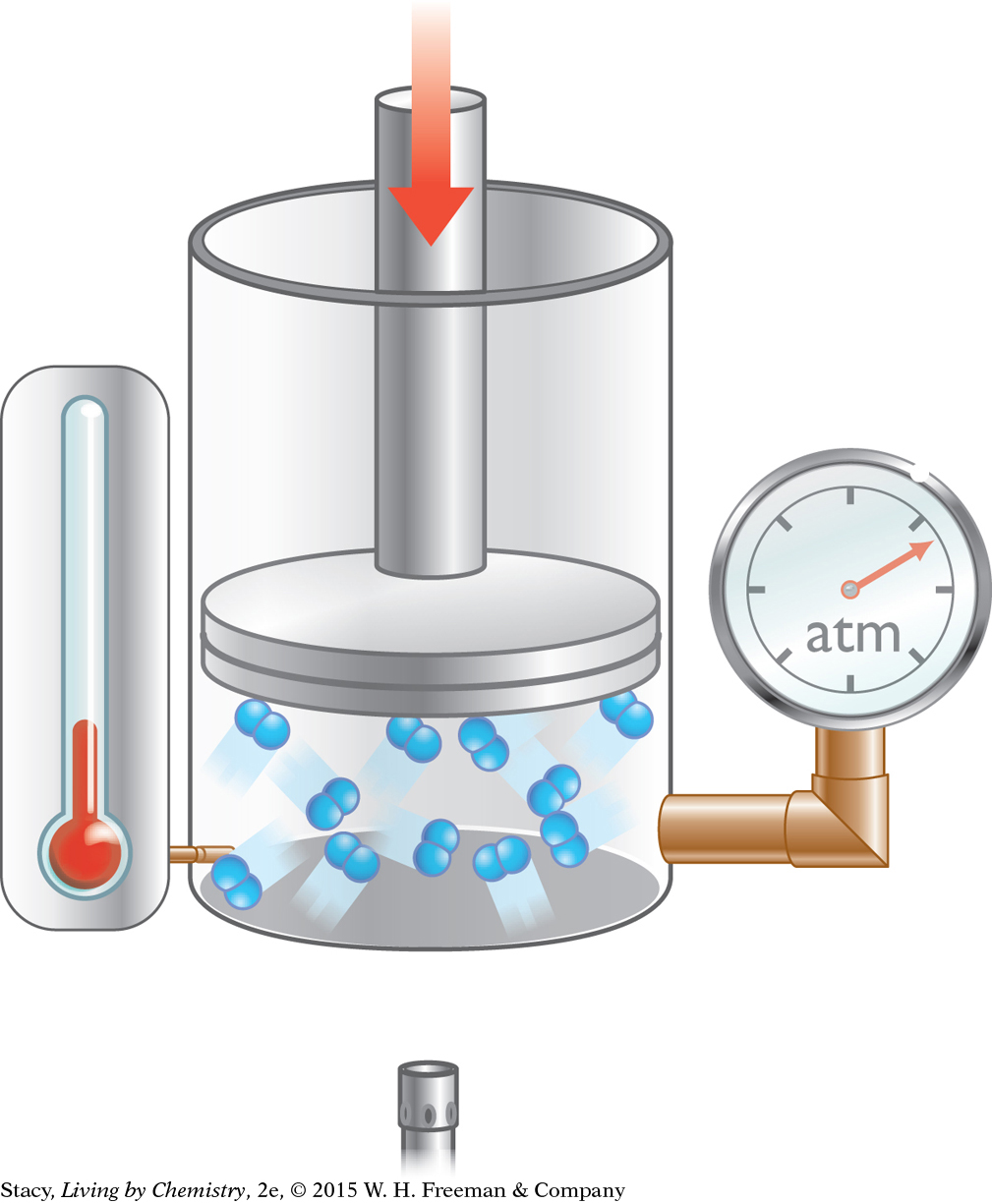
Action: The piston is pushed downward to decrease the volume. The temperature is held constant by allowing heat to exchange with the surroundings.
Observation: The gas pressure increases.
Explanation: When the gas molecules are forced into a smaller space, they hit the walls of the container more often. The gas pressure is higher. This change is associated with Boyle’s law.
CHANGING TEMPERATURE
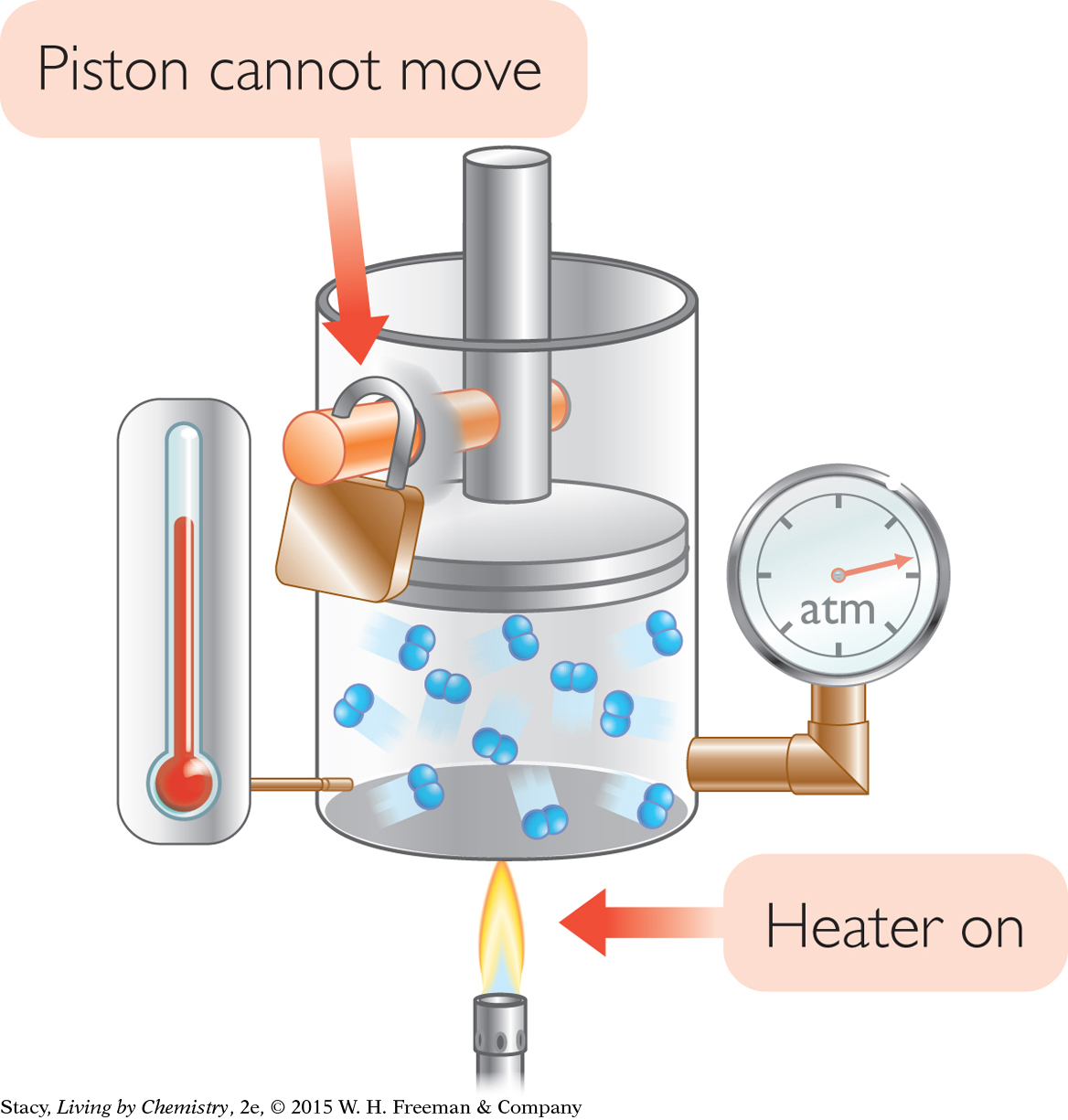
Action: The gas is heated. The volume is held constant by clamping the piston so that it cannot move.
Observation: The gas pressure increases.
Explanation: At higher temperatures, the gas molecules move faster. They hit the walls of the container harder and more often, which increases the gas pressure. This change is associated with Gay-Lussac’s law.
LESSON SUMMARY
LESSON SUMMARY
How do molecules cause gas pressure?
KEY TERM
kinetic theory of gases
316
The collisions of gas molecules with the walls of a container cause gas pressure. If the volume decreases while the temperature stays the same, the molecules collide with the walls more frequently. This causes an increase in gas pressure. If the volume stays the same and the temperature increases, the molecules move faster. They collide with the walls more frequently and they hit the walls harder. This causes an increase in gas pressure.
Exercises
Reading Questions
Use the kinetic theory of gases to explain why increasing the gas volume decreases the gas pressure.
Use the kinetic theory of gases to explain why decreasing the gas temperature decreases the gas pressure.
Reason and Apply
Suppose that the pressure of a gas in a cylinder has increased. What might have changed to cause this? Explain your thinking.
Why is it dangerous to heat a gas in a sealed container?
Can you decrease the volume of a gas to zero? Why or why not?
When you fly in a commercial airplane, you often feel the change in air pressure in your ears. It feels painful. Use the kinetic theory of gases to explain what you think is happening.
At sea level, the pressure of trapped air inside your body is 1 atm. It is equal to the pressure of air outside your body at sea level. Imagine that you do a deep sea dive. You descend slowly to a depth where the pressure outside your body is 3.5 atm.
How does the volume of your lungs compare at 3.5 atm with the volume at sea level?
Why is it dangerous to hold your breath and ascend quickly?
As a helium balloon floats up into the sky, the pressure of the atmosphere around it decreases as the balloon’s altitude increases. If the gas temperature does not change, what do you expect will happen to the volume of the balloon? Explain your thinking.
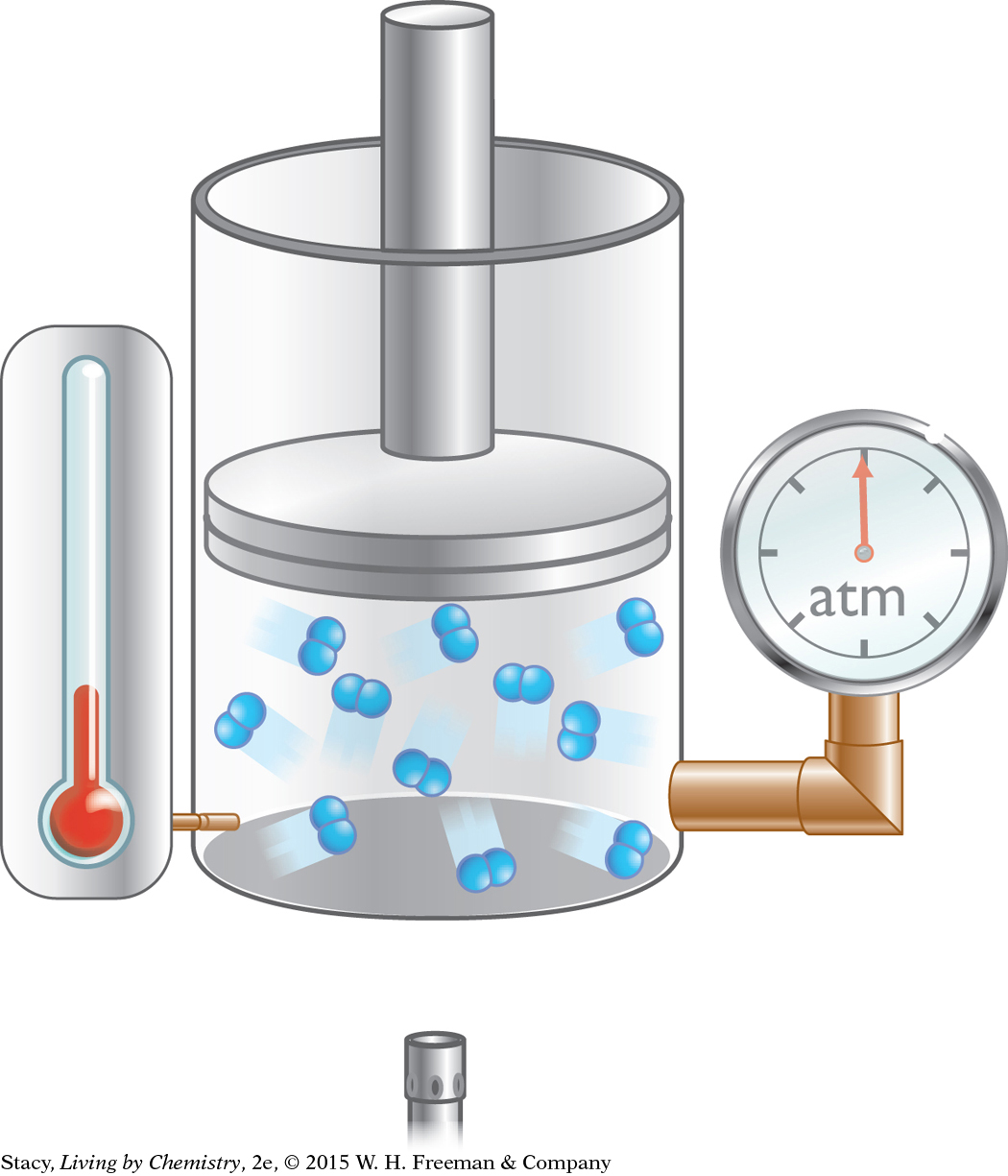
A gas is trapped in a cylinder with a piston as shown. The gas pressure is 1.0 atm, the volume is 500 mL, and the temperature is 300 K.
Draw the cylinder after the volume has been changed to 1000 mL. The gas temperature is 300 K.
Does the pressure inside the cylinder increase or decrease? Explain your thinking.
Which gas law applies?
Look up information on steam engines in a book or on the Web. Explain how a steam engine works.