LESSON 63: n/s for Number: Pressure and Number Density
327
THINK ABOUT IT
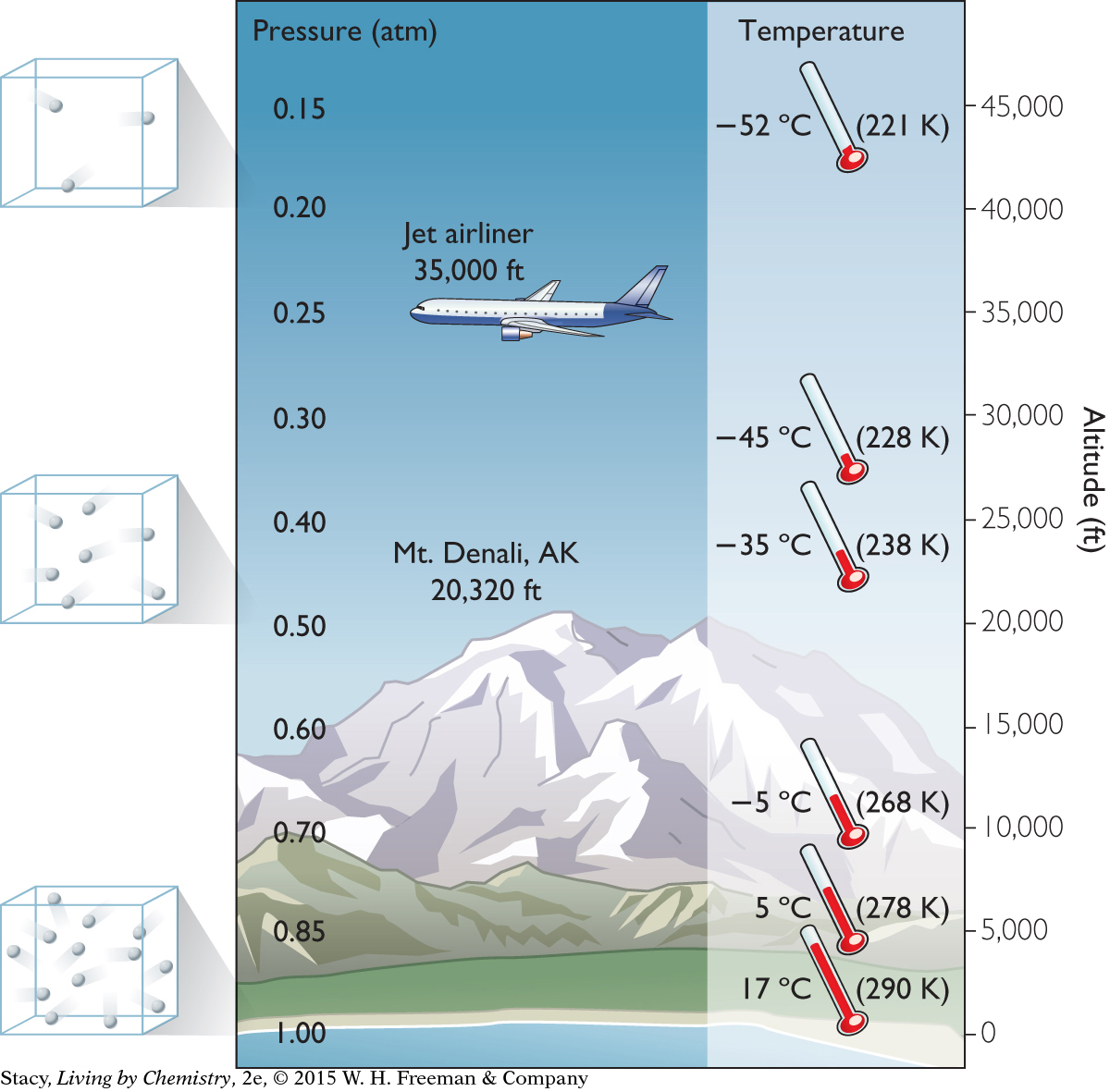
Airplanes fly at about 35,000 ft (~6.6 mi) above Earth. At this altitude, the air pressure is less than 0.25 atm and the air temperature is less than –45 ºC. It is very cold, and the air is very thin up there! You might wonder how many oxygen molecules are available at this air pressure to breathe.
How is the number of gas molecules in a sample related to pressure?
To begin to answer this question, you will explore
Pressure and Number Density
Measuring Air Pressure
Pressure and Number Density
EXPLORING THE TOPIC
Pressure and Number Density
The illustration provides information on the troposphere, or lower atmosphere, where clouds form and weather takes place. The containers with spheres show how the density of air molecules decreases with altitude.
328
HISTORY CONNECTION
HISTORY
CONNECTION
The “weather glass” is an old-fashioned instrument for predicting the weather. The liquid is higher in the spout than in the bottle. This indicates that the atmospheric pressure is lower than average and a storm is approaching.
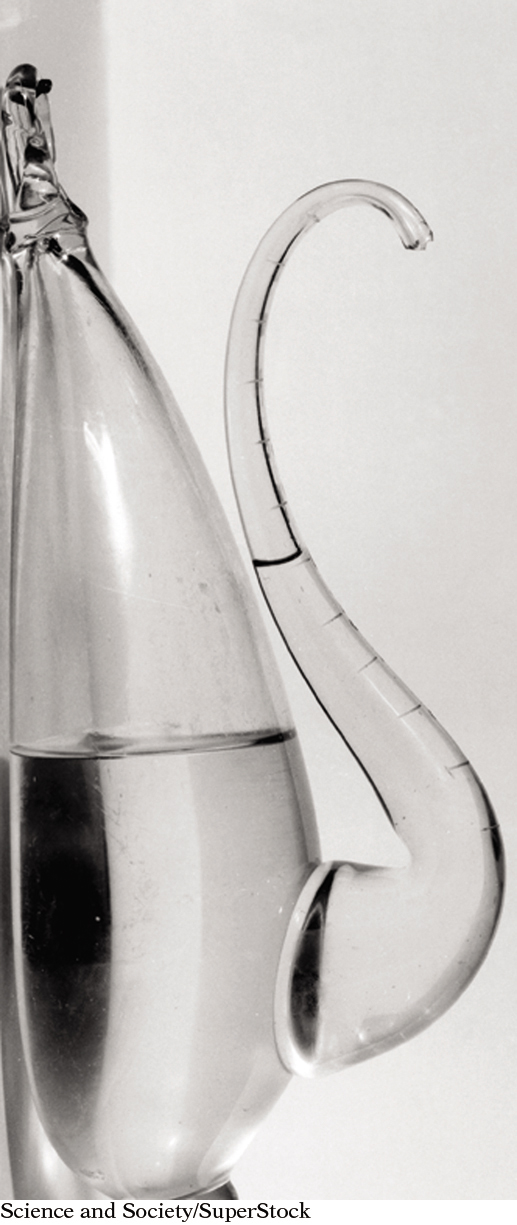
Chemists use the variable n to refer to the number of molecules, atoms, or particles they are considering. The number of air molecules per unit of volume is referred to as the number density, n/V.
The number density of air molecules is relatively low at high altitudes. This is why it gets more difficult to breathe at higher altitudes. There are fewer air molecules per volume of air.
If there are fewer gas molecules in a certain volume, then the air pressure will be lower. Air pressure is proportional to number density. This relationship can be expressed by the equation
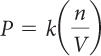
The decrease in air pressure with increasing altitude is due mainly to a decrease in the number density of gas molecules.
Big Idea
Big Idea
The more gas molecules you have in a space, the greater the gas pressure will be.
Example
Bicycle Tire
Use the kinetic theory of gases to explain why the air pressure increases as you pump up a bicycle tire.
Solution
As you pump up a tire, the number of gas molecules per unit of volume becomes greater and greater. This increases the number of collisions between molecules and the walls of the tire. The pressure inside the tire increases until the tire is almost rigid.
Measuring Air Pressure
Measuring Air Pressure
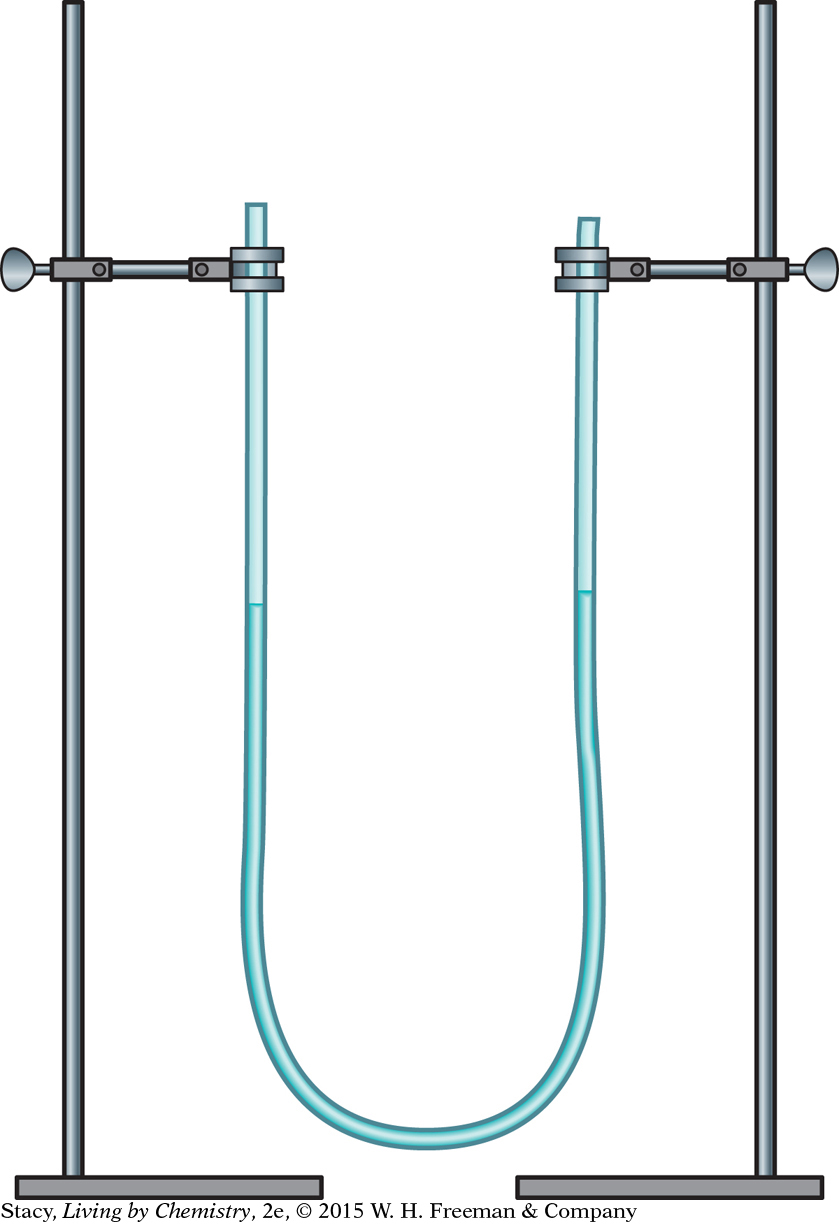
There are a number of different ways to measure air pressure. Meteorologists and chemists commonly use either a manometer or a barometer. Both instruments measure the height of a liquid in a tube.
MANOMETER
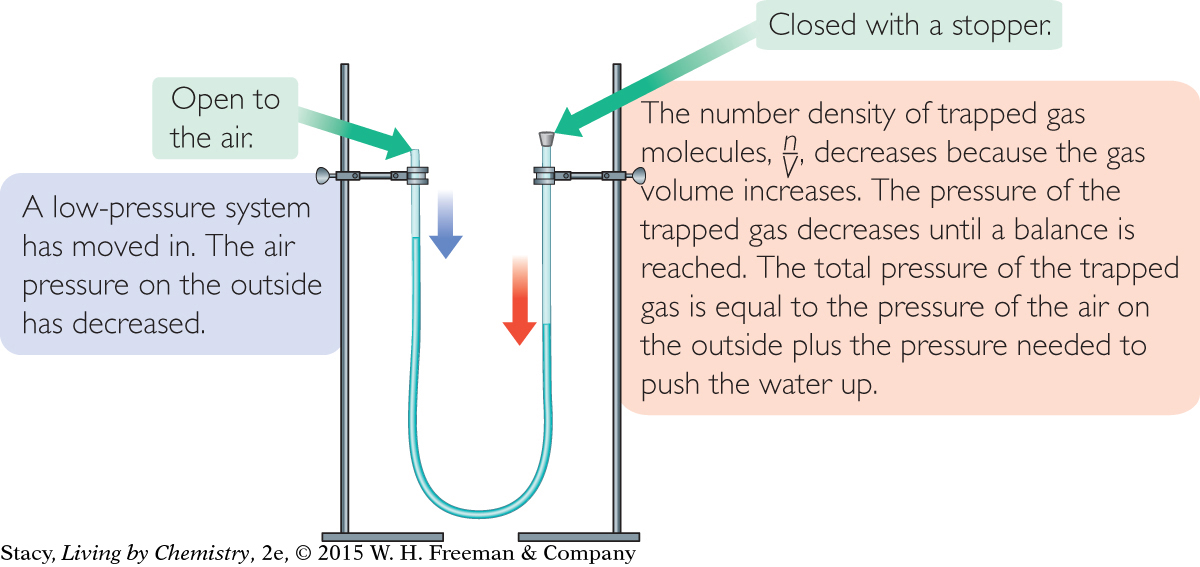
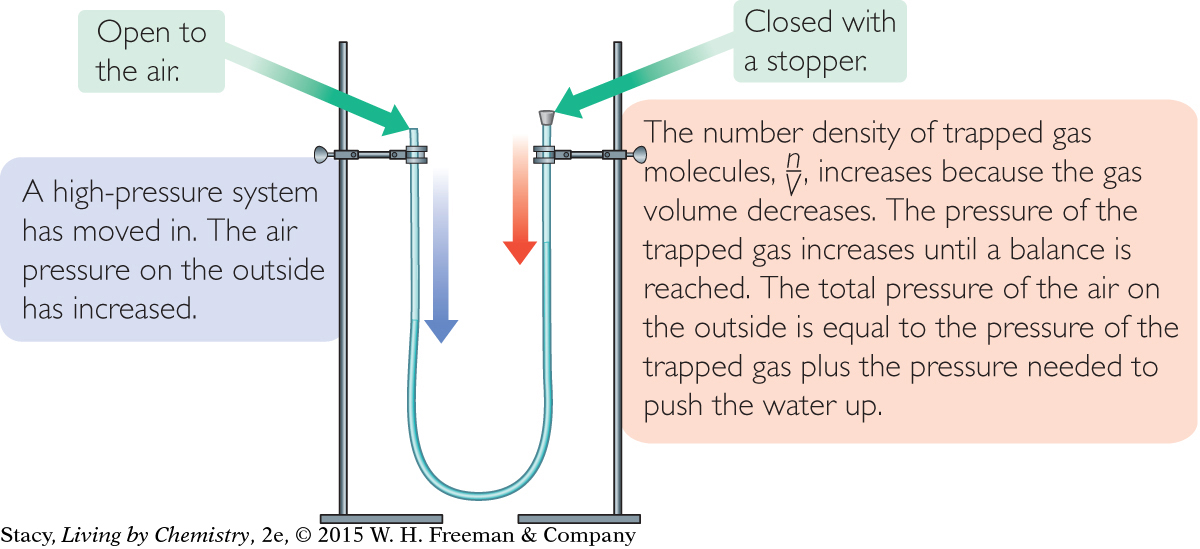
A manometer consists of a liquid in a U-tube as shown in the illustration. If the U-tube is partially filled with water, the water levels on both sides of the U will be at equal heights. This is because the air pressure is the same on both sides.
Imagine that you seal off one side of the U-tube with a stopper so that the number of molecules in the sealed-off area cannot change. The heights of the water will be the same, at least at first. However, if the outside air pressure increases, it will push on the water in the open end causing the water to move up on the side with the stopper, compressing the air on that side. The air pressure of the trapped gas increases because n/V increases. If the outside air pressure decreases, the water in the open end will rise. The air pressure of the trapped gas also decreases because n/V is smaller.
329
HISTORY CONNECTION
HISTORY
CONNECTION
Evangelista Torricelli invented the barometer. Pressure is still sometimes reported in torrs. A torr is equivalent to a millimeter of mercury, or mm Hg. 1 atm = 760 mm Hg = 760 torr

In a U-tube manometer, the water level on the closed side rises when the air pressure in the atmosphere increases. The water level on the closed side falls when the air pressure in the atmosphere decreases.
BAROMETER
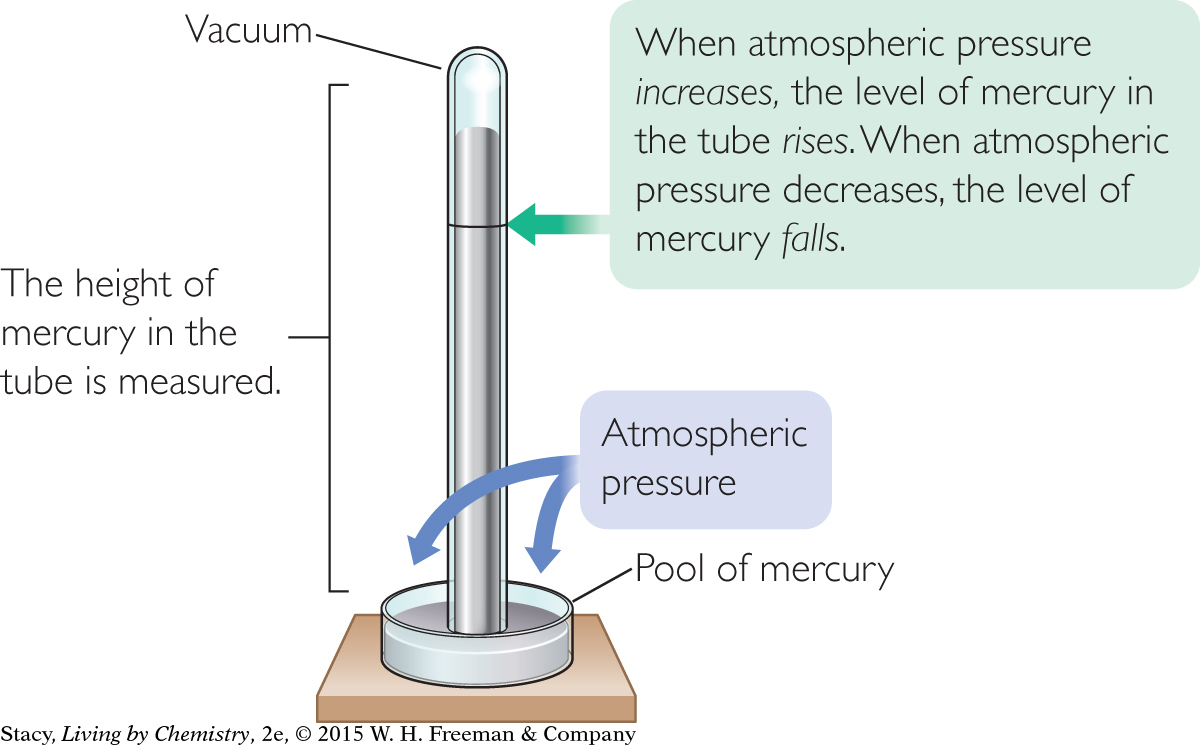
A barometer consists of a tube filled with liquid, which is inverted in a dish of the same liquid. The surface of the pool of liquid is exposed to the atmosphere. There is almost no gas pressure inside the tube.
The barometer shown in the illustration measures the difference in pressure on two surfaces of a liquid. The pressure on the liquid inside the tube is close to zero, so it is the mass of liquid in the tube that balances the air pressure on the outside.
Important to Know
The liquid does not spill out of the inverted tube because the force of the air pushing on the surface of the liquid is greater than the weight of the liquid pushing down in the inverted tube.
330
Mercury is often the liquid of choice for barometers due to its high density. Air pressure is sometimes expressed in inches of mercury or millimeters of mercury.
1 at = 29.9 in Hg = 760 mmHg
Meteorologists will sometimes say that the mercury is rising when a high-pressure system arrives, or that the mercury is falling when a low-pressure system moves in. They are referring to the motion of the liquid mercury in the inverted tube.
LESSON SUMMARY
LESSON SUMMARY
How is the number of gas molecules in a sample related to pressure?
KEY TERM
number density
Number density is a measure of the number of molecules, atoms, or particles per unit of volume, n/V. The number density of air molecules in the atmosphere decreases with increasing altitude resulting in lower gas pressure. It is possible to monitor atmospheric pressure by monitoring the level of a liquid in a tube that is sealed at one end. Manometers and barometers are two instruments that measure gas pressure.
Exercises
Reading Questions
How can you account for the decrease in air pressure with increasing altitude?
Use the kinetic theory of gases to explain the relationship between number density and gas pressure.
Explain the difference between a manometer and a barometer.
Reason and Apply
What effect does changing the number of gas particles in a container have on the gas pressure in the container? Use the kinetic theory of gases in your explanation.
Name three ways that you can increase the pressure of a tire.
The boxes in the illustrations show tiny samples of air. Assume they are at the same temperature.
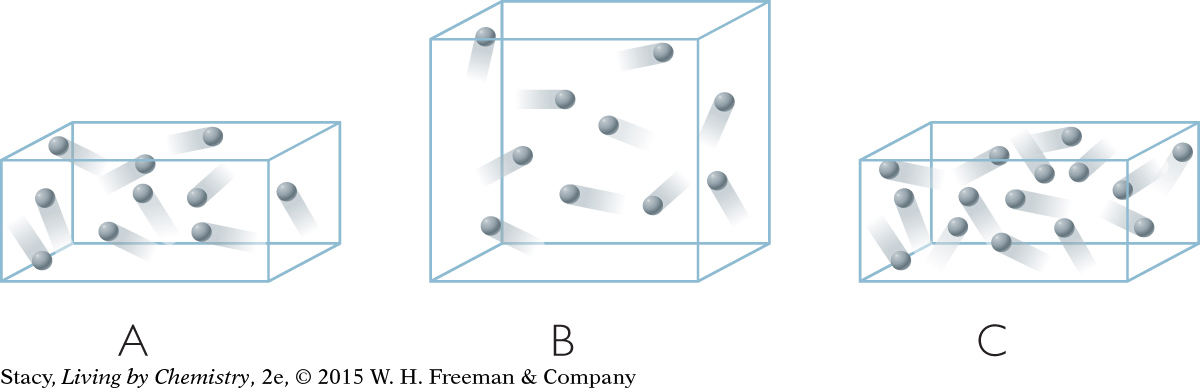
List the samples in order of increasing gas pressure. Explain your reasoning.
Sketch a volume of air that has a pressure in between the pressures in boxes A and B.
Explain how the mercury level in a barometer changes when a high-pressure system moves into a region.
Explain why the liquid in the barometer does not spill out.