LESSON 66: Feeling Humid: Humidity, Condensation
339
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
Only mammals sweat. Humans and other primates have sweat glands all over their bodies. Dogs, cats, and mice have sweat glands only on their feet. Rabbits have sweat glands around their mouths. Dogs pant when they are hot, and the evaporation cools them down. Cats lick their paws and chests. The water evaporates from their fur, cooling them.

THINK ABOUT IT
Exercising can make you work up a sweat. Sweating helps to regulate your body temperature. When a breeze blows across your sweaty forehead, you may notice that your skin feels cooler. However, on a humid day, with a lot of moisture in the air, it seems as if no amount of sweating helps you to cool down.
What is humidity and how is it measured?
To answer this question, you will explore
Evaporation and Condensation
Humidity
Relative Humidity
Evaporation and Condensation
EXPLORING THE TOPIC
Evaporation and Condensation
Important to Know
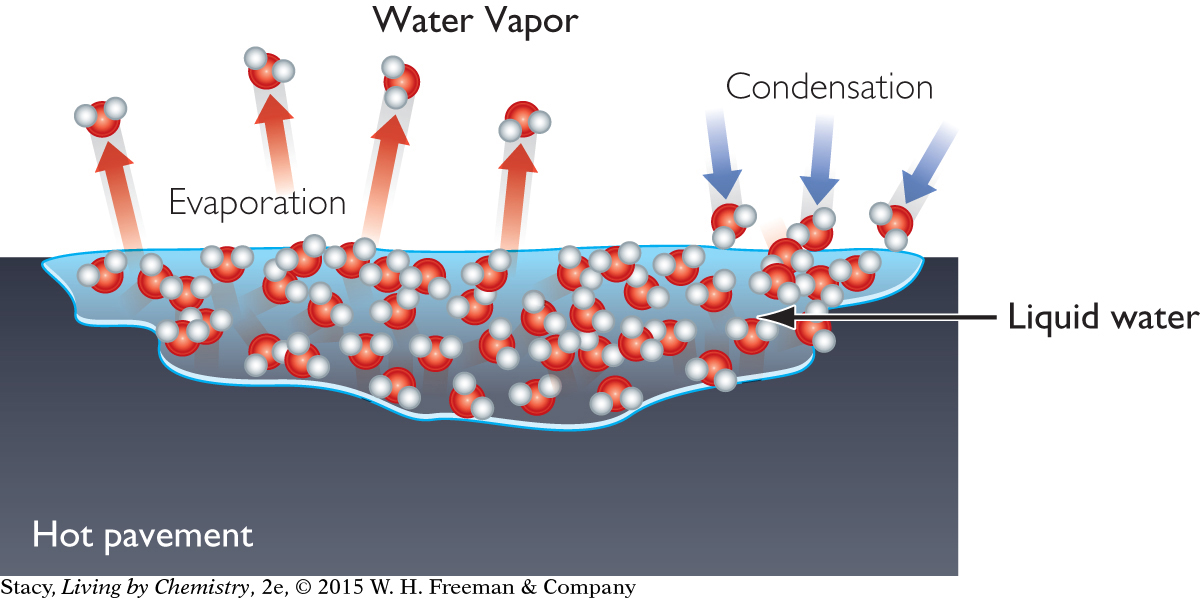
The water in rain puddles does not have to boil to go into the gas phase. Evaporation takes place on the surface of a liquid all the time. Try leaving a glass of water on a table. The water will gradually disappear, leaving the glass empty.
After a summer rainstorm, puddles of water on the ground often disappear quickly. The rainwater is evaporating. Recall that evaporation is a phase change from a liquid to a gas.
Evaporation is the reverse process of condensation, when water vapor becomes a liquid. These two processes, evaporation and condensation, are both occurring wherever water is present. In fact, there is a competition between the two processes that can result in net evaporation or net condensation.
The rates of both evaporation and condensation depend mainly on temperature and the amount of water vapor already in the air.
GEOLOGY CONNECTION
GEOLOGY
CONNECTION
It is estimated that Earth has about 326 million cubic miles of water. This includes all of the water in the oceans, underground, and locked up as ice. The U.S. Geological Survey estimates that about 3100 mi3 of this water is in the air—mostly as water vapor, but also as clouds or precipitation—at any one time.

Cloud formation, rain and snowfall, fog, frost, and the appearance of dew are all events associated with the condensation of water vapor out of the atmosphere. You might have noticed a tendency for fog to form on cool nights after a warm, humid day. This is because a lot of water has evaporated during the day. When the temperature decreases, the amount of water vapor in the air exceeds the maximum allowable for the cooler temperature and fog forms.
Humidity
Humidity
340
Water vapor is present in the air around us all the time. There is more or less water vapor in the air depending on the weather conditions. Warmer air masses can have more water vapor than colder air masses.
METEOROLOGY CONNECTION
METEOROLOGY
CONNECTION
Weather forecasters use a sling psychrometer to determine the relative humidity. It consists of two thermometers, one dry and one wrapped in a wet cloth. The thermometers are spun through the air and then read.
During spinning, water evaporates from the cloth, cooling the wet-bulb thermometer. The amount of cooling that occurs is directly related to how much moisture is in the air. When there is more moisture in the air, less evaporation and less cooling occur.

Humidity is the number density of water vapor in the air in moles per unit of volume, or n/V. Sometimes, it is expressed as grams per unit of volume (such as cm3 or liters). For a given temperature, there is a limit to the number density of water molecules that can be in the air. At a certain point, the air becomes saturated, and no further net evaporation takes place. The graph shows the maximum number density for air temperatures between –1 ºC and 40 ºC. You can see that the maximum humidity depends on temperature.
On a cool day, around 10 ºC (50 ºF), for instance, the maximum number density of water molecules in the air is approximately 0.5 mol per 1000 liters of air. This amount represents the maximum humidity for this temperature. The actual humidity may be at or below this value.
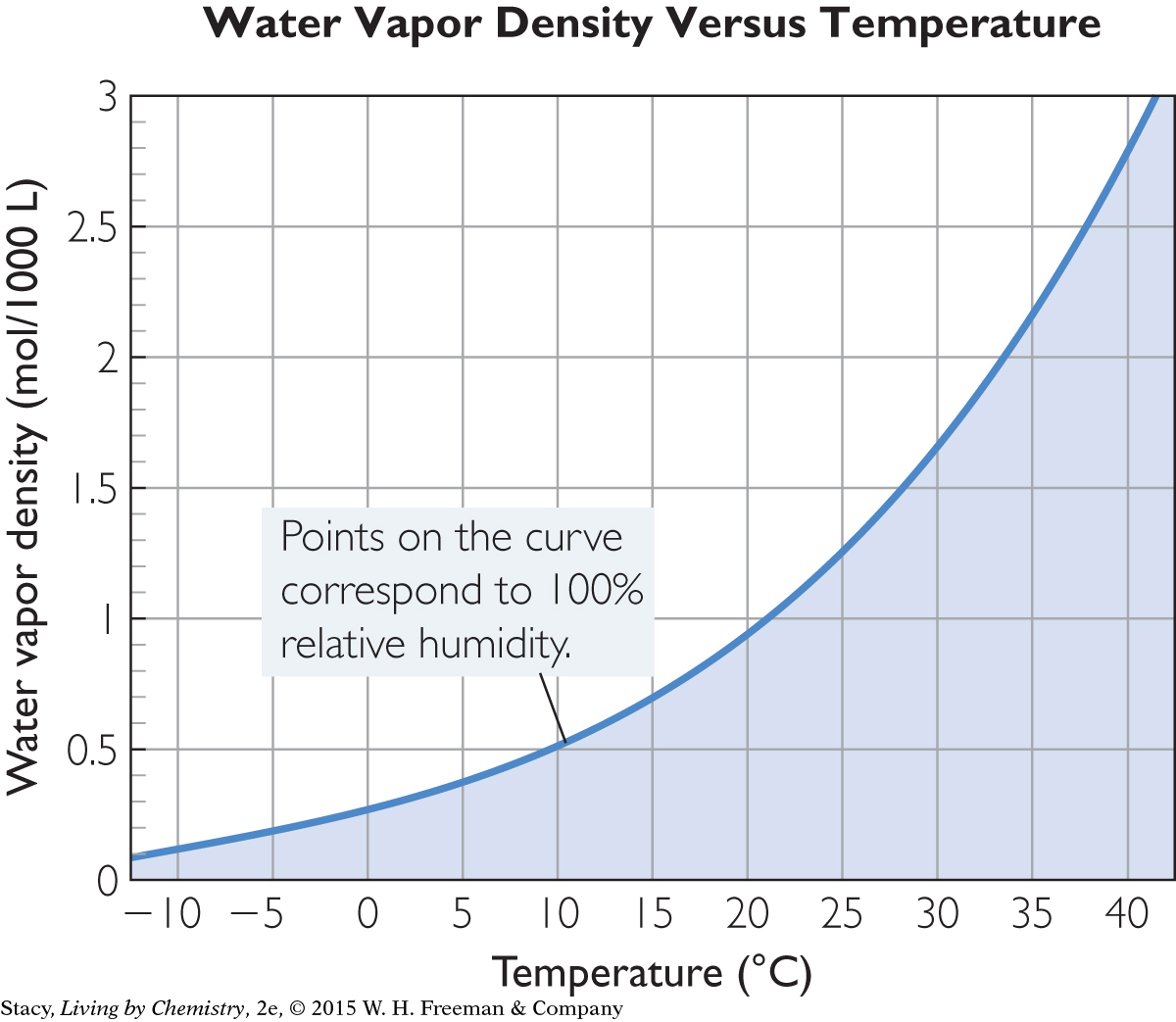

If the temperature of the air drops or the humidity rises, precipitation can occur. If these two values, T and n/V, result in a data point above the curve, there will be precipitation or condensation of some sort. The condensation of water on the outside of a glass of ice water is a sign that the air next to the glass has cooled enough for the water vapor to change phase to a liquid.
341
Like all gases, water vapor exerts a pressure. This pressure is part of the total pressure exerted by all the gases in the atmosphere. Each gas exerts a partial pressure, and these partial pressures add up to the atmospheric pressure.
Relative Humidity
Relative Humidity
When meteorologists consider humidity, they focus on the relative humidity. Relative humidity is the percent of the maximum humidity for a specified temperature. When the air contains the maximum amount of water vapor for the temperature of the air, it is at 100% relative humidity.
The terms humidity and relative humidity are often used interchangeably in weather reports. Meteorologists might say the humidity today is at 35% when they are really talking about the relative humidity.
Example
Relative Humidity
Imagine that on a certain day the water vapor density is 1.6 mol/1000 L and the temperature is 30 ºC.
What is the relative humidity?
At night, the temperature drops to 20 ºC. Do you expect there will be precipitation?
Solution
You can read the maximum water vapor density at 30 ºC on the graph Water Vapor Density Versus Temperature. It is 1.7 mol/1000 L. This corresponds to 100% humidity. The relative humidity is the measured water vapor density divided by the maximum possible water vapor density.
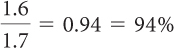
The point (20 ºC, 1.6 mol/1000 L) on the graph is above the 100% humidity curve, so you can expect precipitation.
Matter that is in contact with an evaporating liquid will cool off. Sweating helps cool you because the evaporation of water on your skin transfers heat away from the body. When there is more water vapor in the air, it becomes harder for sweat to evaporate. This is why you often feel much hotter in humid air than in dry air.
342
LESSON SUMMARY
LESSON SUMMARY
What is humidity and how is it measured?
KEY TERMS
humidity
partial pressure
relative humidity
Humidity refers to the amount of water vapor in the air. It is a measure of the number density of water molecules. Air temperature affects how much water vapor can be in the air. Warmer air can contain more water vapor than colder air. There is an upper limit to the amount of water vapor that can be in the air at any given temperature. This upper limit is called maximum humidity, or 100% relative humidity. At 100% relative humidity, no more water can evaporate into because the air is saturated. You might expect rain or fog when the relative humidity is close to 100%.
Exercises
Reading Questions
What does humidity measure?
Explain what is meant by relative humidity.
Reason and Apply
What is the maximum vapor density possible at 30 °C?
Is it possible for the water vapor density in the air to reach 10 mol per 1000 L at a temperature of 40 °C? Explain why or why not.
Use the graph Water Vapor Density Versus Temperature to predict the water vapor density at 100% humidity for 35 °C.
What is the relative humidity if there is 0.5 mol of water vapor per 1000 L of air at 40 °C?
When the humidity is 25% and the temperature is 30 °C, what is the water vapor density?
On one day, 100% humidity corresponds to 1.9 mol of water vapor per 1000 L of air. On another day, 100% humidity corresponds to only 1.0 mol of water vapor per 1000 L of air. How can two different water vapor densities both be at 100% humidity?
Explain why it is easy to get dehydrated when you are exercising at a temperature of 0 °C and a relative humidity of 20%.
Do you feel cooler at 100% humidity at 30 °C, or at 50% humidity at 30 °C? Explain your thinking.
Suppose the humidity is 65% during the day when the temperature is 30 °C.
If the temperature drops to 25 °C at night, do you expect fog? Explain.
If the temperature drops to 20 °C at night, do you expect fog? Explain.
On a cold winter day the relative humidity is 50% outdoors but only 5% indoors. Which answer best explains what is going on?
It must be raining outside.
It must be snowing outside.
The heater is on inside, so the same humidity is a lower relative humidity.
The heater is on inside, and it is evaporating some of the humidity in the air.