LESSON 68: Toxic Reactions: Chemical Equations
355
THINK ABOUT IT
In the world around you, matter undergoes many changes. Some of these changes have very little impact on living things on the planet. Other changes may be vital and necessary for life. Still other changes may threaten the health and well-being of plant and animal life. Chemists use chemical equations to keep track of all types of changes in matter, including those that are beneficial and those that are unsafe.
How do chemists keep track of changes in matter?
To answer this question, you will explore
Chemical Equations
Toxic Substances and Their Effects
Chemical Equations
EXPLORING THE TOPIC
Chemical Equations
A chemical equation is a chemical “sentence” that describes change, using numbers, symbols, and chemical formulas. Chemical equations describe what happens when a single substance is changed, or when two or more substances are combined and a change occurs. Once you understand how to decode chemical equations, you will be able to use them to predict what you might observe when substances are mixed.
INTERPRETING A CHEMICAL EQUATION
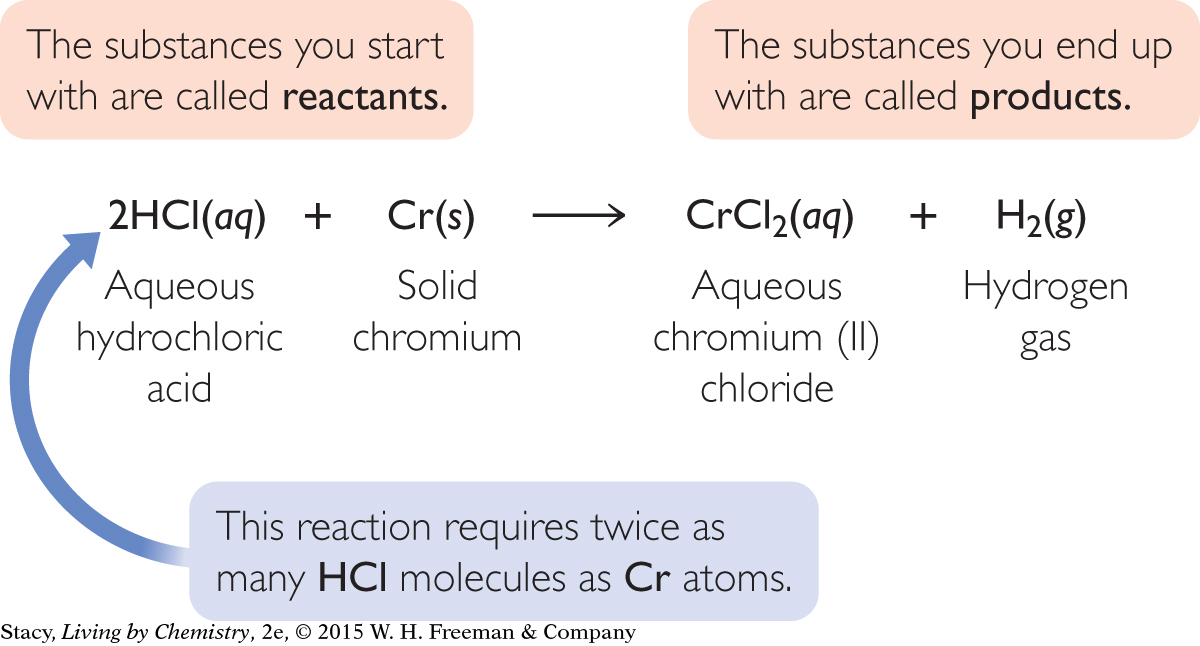
In some reactions that take place in your body, the element chromium is safe and even necessary. In other reactions, it is toxic. Consider what happens if you ingest chromium metal and it reacts with the hydrochloric acid in your stomach. A chemical equation can help you decode this reaction.
356
ENVIRONMENTAL CONNECTION
ENVIRONMENTAL
CONNECTION
In larger amounts, some chromium compounds cause cancer. Since the 1980s, Erin Brockovich has brought several successful lawsuits in California to stop unsafe levels of chromium compounds in drinking water. A legal clerk at the time of her first legal case, she is now a consultant and speaker.

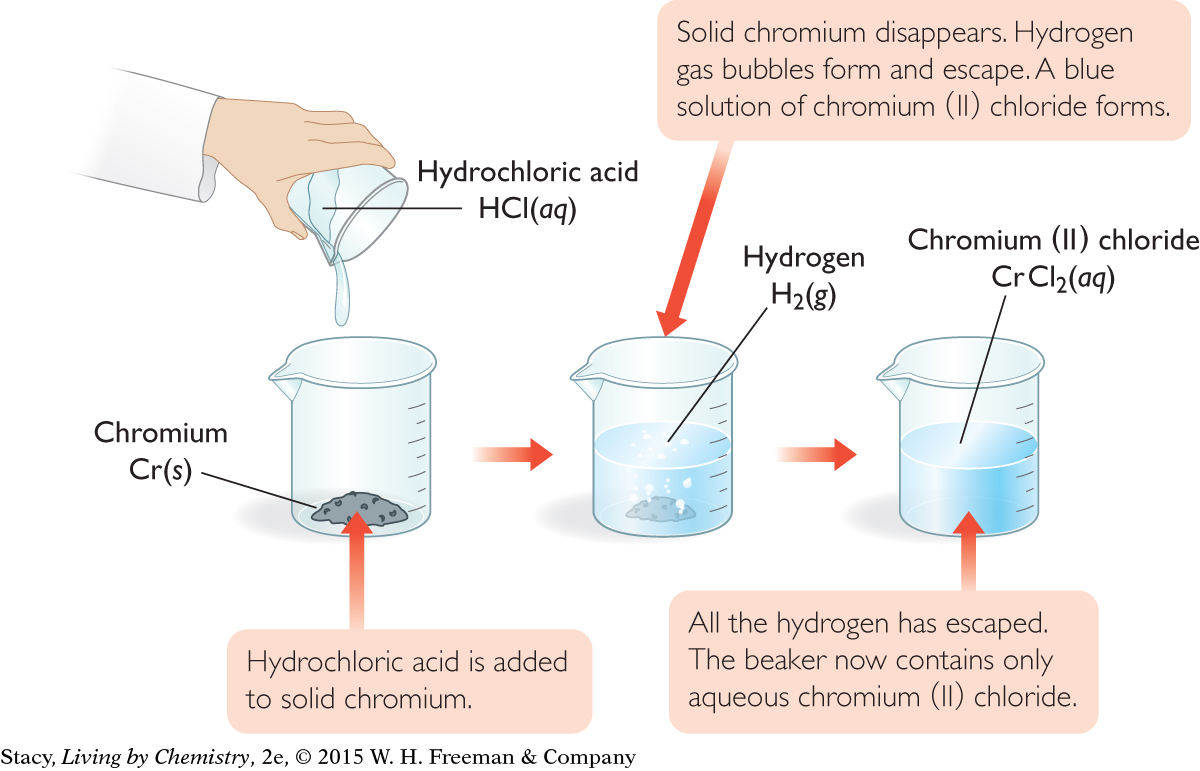
Interpretation: Hydrochloric acid reacts with solid chromium to produce a solution of chromium (II) chloride and bubbles of hydrogen gas.
A chemical equation can help you anticipate what you will observe when the reactants are combined. Examine the same equation, but focus on what you expect to observe.
2HCl(aq) + Cr(s) → CrCl2(aq) + H2(g)
According to the equation, when hydrochloric acid and chromium react, the solid chromium will disappear. You would expect to see the formation of a new aqueous solution as well as some evidence that a gas was produced.
Sometimes, the changes that take place when substances are mixed are not visible to the eye. For example, death from poisoning occurs within minutes of swallowing a solution of sodium cyanide, NaCN. One successful treatment for this type of poisoning is injection of an antidote, a remedy that counteracts the poison. The antidote for sodium cyanide is a solution containing sodium thiosulfate, Na2S2O3. But if you were to observe the reaction between sodium cyanide and sodium thiosulfate in a beaker, you would not be able to tell that a reaction had occurred because both reactants and both products are clear, colorless liquids.
Big Idea
Big Idea
Chemical equations keep track of changes in matter.
Toxic Substances and Their Effects
Toxic Substances and Their Effects
Toxic substances enter the body in limited ways. The most common methods are through ingestion (eating or swallowing), inhalation (breathing something in), or contact with the skin. Once toxic substances enter the body, they react in a variety of ways.
Toxic substances can react with water in mucous membranes, with oxygen carried through the blood, or with stomach acid. Some toxic substances have an immediate negative effect on the well-being of a person or living thing. Other toxic substances may stay a long time in the body, becoming part of the body’s chemistry, perhaps damaging the body many years later.
357
Toxic substances may be molecular, ionic, or metallic substances. Many small molecular substances react with the body and create acid products, which can damage and irritate tissue or upset the acidity of the blood. Toxic metals often react to form ionic compounds, which move throughout the body and compete with “good” metals that are useful to the body. Some ionic compounds form solids that clog the body’s filtering systems. Of course, these are just a few of the possible types of toxic reactions that occur. Chemical equations are the main tool you will use to track these changes.
LESSON SUMMARY
LESSON SUMMARY
How do chemists keep track of changes in matter?
A chemical equation tracks changes in matter. The left side of the equation contains the chemical formulas for the reactants or the substances that are being combined. The right side of the equation contains the chemical formulas for the products or the substances that are produced. The equation also shows the phase of each reactant and product. Decoding an equation allows you to predict what substances may be made when the reactants are combined. Chemical equations often provide more information than what you can observe with your senses.
Exercises
Reading Questions
What is the difference between a reactant and a product?
Are chemicals and chemical reactions important for life? Why or why not?
Describe in your own words what a toxic substance is.
Reason and Apply
Both bleach and ammonia are used for cleaning. However, it is very dangerous to mix bleach with ammonia because they react to produce sodium hydroxide and the toxic gas chloramine.
NaOCl(aq) + NH3(aq) → NaOH(aq) + NH2Cl(g)
Write an interpretation of the chemical equation.
What do you expect to observe?
Poisoning with mercury chloride can be reversed by chelation therapy. The chelating agent called EDTA, C10H16N2O8, is injected into the bloodstream. EDTA forms a water-soluble compound with mercury ions, allowing removal from the body through the kidneys.
HgCl2(s) + C10H16N2O8(aq) → HgC10H12N2O8(aq) + 4HCl(aq)
Write an interpretation of the chemical equation.
What do you expect to observe?
Describe at least three types of effects that a toxic substance can have on the body.