LESSON 69: Making Predictions: Observing Change
358
THINK ABOUT IT
Table salt, or sodium chloride, NaCl, can enhance the flavor of soup. Even though the salt dissolves in the soup, you can still taste it. This change is described by a chemical equation:
NaCl(s) → NaCl(aq)
It is also possible to change the salt in far more dramatic ways. For example, when an electric current is passed through a sodium chloride solution, a toxic, green gas bubbles out of the solution. The change is described by this chemical equation:
2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(g) + H2(g)
How can you predict what you will observe based on a chemical equation?
To answer this question, you will explore
Predicting Change from Chemical Equations
Information in Chemical Equations
Predicting Change from Chemical Equations
EXPLORING THE TOPIC
Predicting Change from Chemical Equations
While some chemical changes are difficult to see, many changes in matter are accompanied by observable evidence. For example, when a change takes place, you may hear fizzing, a pop, or an explosion. You might see changes in color or physical form. You may smell gases that escape, or you may feel heat. Perhaps there is even a fire.
But what if you don’t have a laboratory and a lot of chemicals around? How can you predict what you might observe if you are provided only with a chemical equation?
SUGAR DISSOLVING
Consider these two chemical equations. What type of change does each describe?
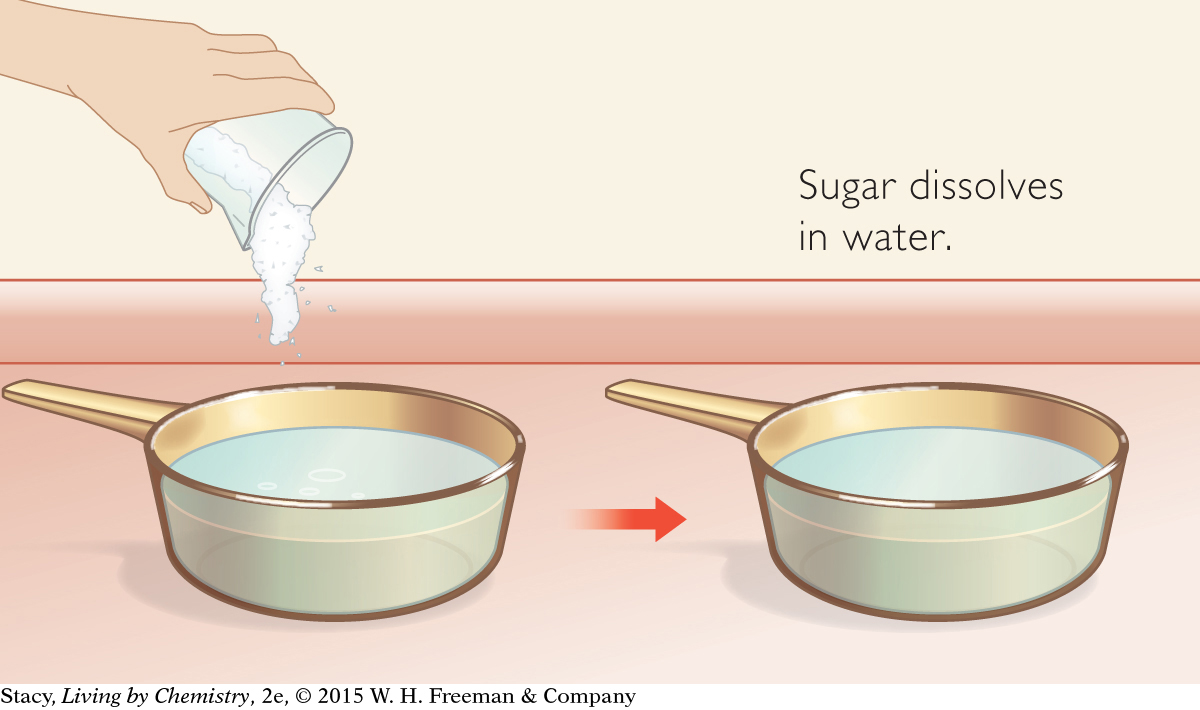
C12H22O11(s) → C12H22O11(aq)
|

C12H22O11(aq) → C12H22O11(s)
|
359
The only difference between these two equations is the position of the (aq) and (s) symbols. Notice that the chemical formula does not change from one side of the equation to the other. The sugar molecule changes form but not identity. The first equation describes dissolving, in which a solid is mixed with water. The second equation describes removing water from an aqueous substance, leaving a solid.
Although the sugar changes appearance when it dissolves, the sugar itself has not changed into a different compound. The chemical equation indicates how the molecules have, or have not, changed.
SUGAR MELTING
Consider these two equations that involve changes to sugar.

C12H22O11(s) → C12H22O11(l)
|

C12H22O11(l) → C12H22O11(s)
|
Again the formulas are identical on both sides of the arrow, except for the symbols in parentheses, (l) and (s). These equations describe a phase change from a solid to a liquid and back to a solid. By heating sugar carefully, you can melt it to a clear liquid. When liquid sugar is cooled, it becomes a solid again.
The same compound shows up on both sides of the equation. Even though the tiny grains of sugar have been transformed into a single, large, chunk of solid sugar, the atoms within the sugar molecules have not rearranged into new compounds. Sugar is still sugar.
Big Idea
Big Idea
When a substance changes phase or dissolves, its chemical formula does not change.
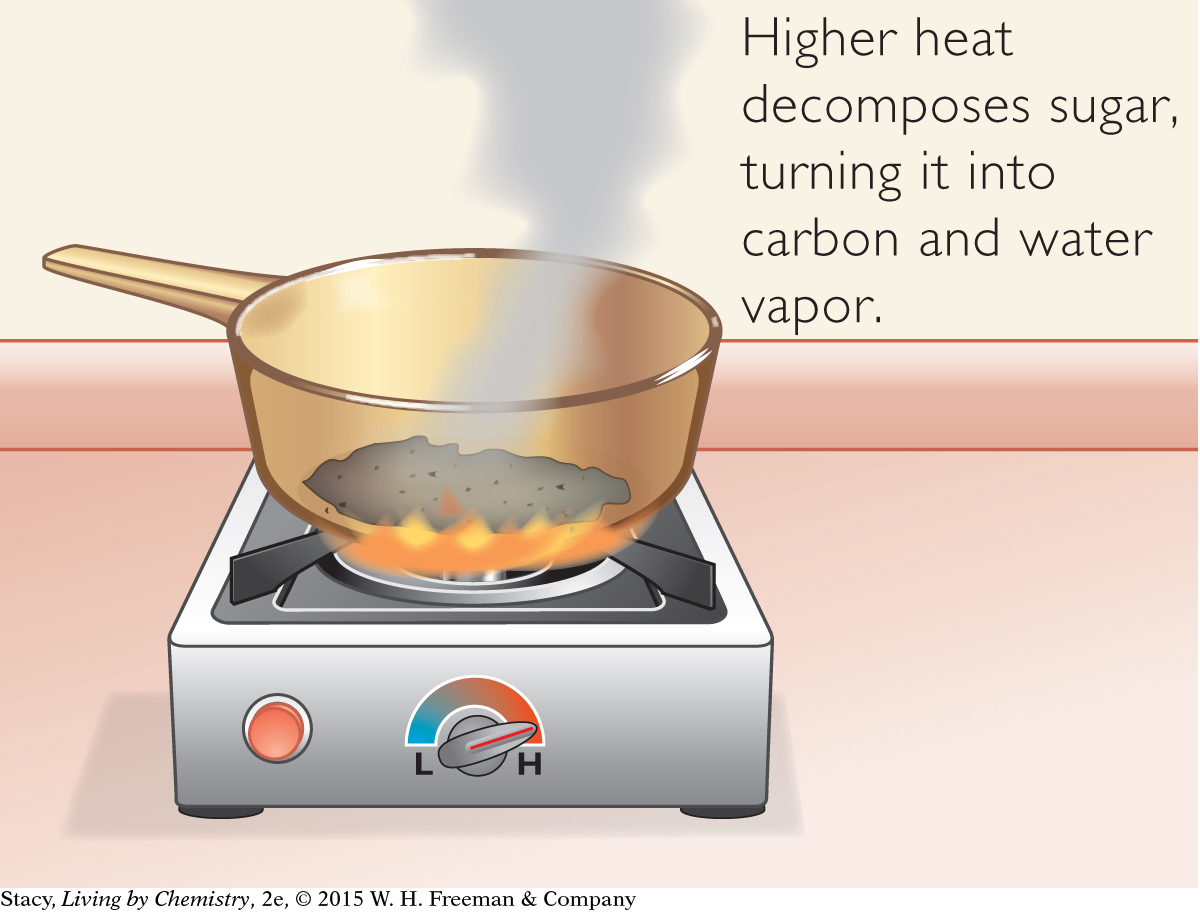
SUGAR DECOMPOSING
Consider another transformation of sugar.
C12H22O11(s) → 12C(s) + 11H2O(g)
Notice that the two sides of the equation look very different. There is no sugar on the right side of the arrow. The sugar has been decomposed. It has been converted into two products: solid carbon and water vapor.
360
If you heat sugar sufficiently (beyond melting), it will turn black and it will release smoke. It will not taste sweet anymore, and there is no way to turn it back into sugar. These property changes, and the release of smoke, are signs that a more dramatic rearrangement of atoms has occurred.
C12H22O11(s) → 12C(s) + 11H2O(g)
|
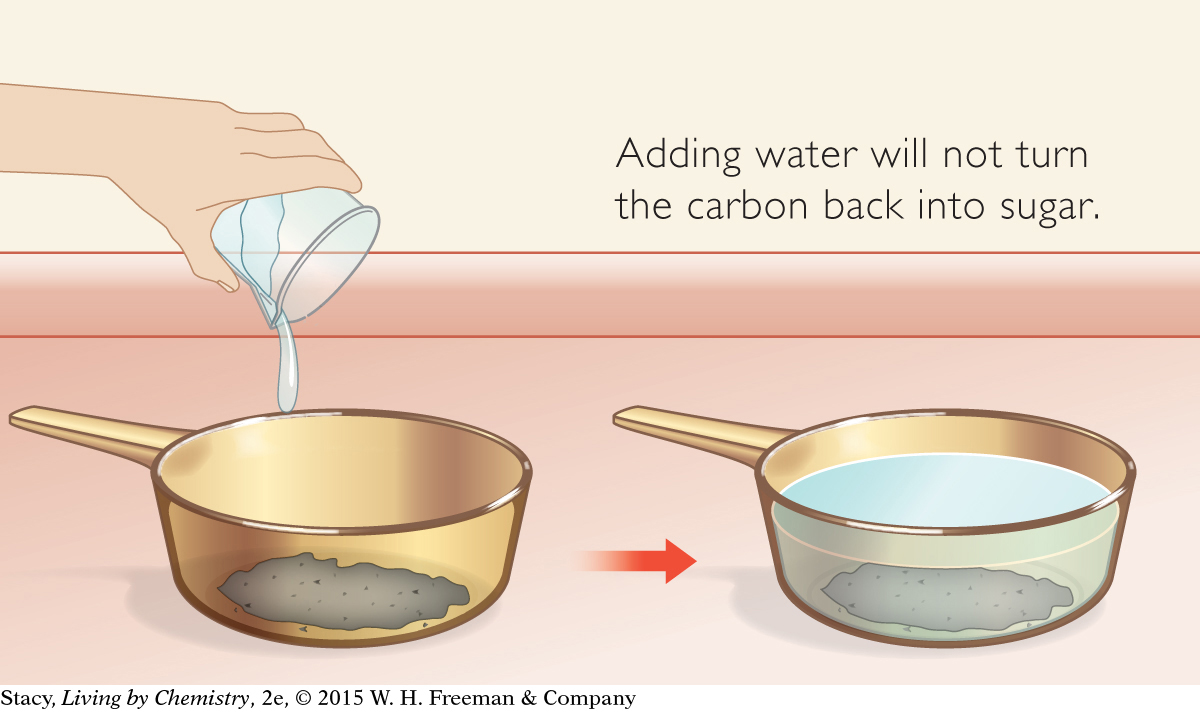
12C(s) + 11H2O(l) → no change
|
This particular rearrangement of atoms is not easy to reverse. In other words, you cannot combine carbon with water and expect to form sugar.
SUGAR REACTING
Consider one more transformation of sugar, as described by this equation:
C12H22O11(s) + 8KClO3(s) → 12CO2(g) + 11H2O(g) + 8KCl(s)
Interpreting the chemical formulas tells you that sugar and potassium chlorate are added together. The result is the formation of two gases, carbon dioxide and water vapor, as well as the formation of some solid potassium chloride.

The sugar and potassium chlorate are converted to carbon dioxide gas, water vapor, and solid potassium chloride. This change happens very quickly and releases energy in the form of light and heat.
361
Example
Changing Salt
Predict what you would observe for the changes described by these equations:
NaCl(s) → NaCl(aq)
NaCl(s) → NaCl(l)
2NaCl(l) → 2Na(l) + Cl2(g)
Solution
The solid sodium chloride dissolves in water.
The solid sodium chloride melts to become liquid sodium chloride.
The liquid sodium chloride decomposes into liquid sodium and chlorine gas.
Information in Chemical Equations
Information in Chemical Equations
CONSUMER CONNECTION
CONSUMER
CONNECTION
When sugar molecules are allowed to crystallize, they form a hard candy, like lollipops. When lemon juice or fructose is added to the sugar, the molecules do not lock into place as in crystalline candy. Instead, they form soft candy like taffy or caramels, called amorphous candy.

Just as a chemical equation can help you predict what you might observe, it can also provide more information than you can get by observation alone. Consider the reaction described at the beginning of this lesson. The change that happens upon passing an electric current through aqueous sodium chloride is described by this equation:
2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(g) + H2(g)
If you were to observe this reaction without knowing the equation, you would not be able to identify the compounds before and after the reaction. Many solutions are clear and colorless, and you might not realize that two gases are produced. A chemical equation identifies the reactants and products in a reaction. This chemical equation indicates that aqueous sodium chloride combines with water to form aqueous sodium hydroxide, chlorine gas, and hydrogen gas. An equation also identifies the form or phase a substance is in. This equation indicates that a liquid of some sort will be observed before and after the reaction, and gas bubbles will be seen as the reaction takes place.
Some information is not contained in a chemical equation. The equation above, for example, does not indicate that chlorine gas is green and toxic. The reaction of sugar with potassium chlorate, mentioned previously, is quite spectacular. The equation does not indicate that the reaction is explosive or that a purple flame is produced. An equation does not tell you whether a procedure will result in hot or cold sensations. It won’t tell you how fast a change will occur or if a color change will be observed. It does not tell you if there will be an explosion, a loud noise, or an overflowing beaker. Only direct observation can provide you with all of the information about what you will see, hear, and feel.
LESSON SUMMARY
LESSON SUMMARY
How can you predict what you will observe based on a chemical equation?
Chemical equations contain valuable information about what is happening during a change in matter. They track the identities of the substances involved in the change, using chemical formulas. They also identify the phase of each substance. By carefully examining a chemical equation, you can predict what you might observe if the procedure were completed. A chemical equation cannot tell you everything that you will observe. It does not inform you about the speed of a reaction, if a color change will occur, or if energy is transferred.
362
Exercises
Reading Questions
In words, describe the difference between sugar melting and sugar dissolving in water. The formula for sugar is C12H22O11.
Use chemical equations to describe the difference between sugar melting and sugar decomposing. The formula for sugar is C12H22O11.
Reason and Apply
Describe what you think you would observe for these chemical equations.
Mg(s) + 2HCl(aq) → H2(g) + MgCl2(aq)
2H2O2(aq) → 2H2O(l) + O2(g)
2NaCl(aq) + Pb(NO3)2(aq) → 2NaNO3(aq) + PbCl2(s)
Write a chemical equation for these reaction descriptions.
Solid sodium chloride dissolves in water.
Solid magnesium sulfide is heated to produce solid magnesium and sulfur gas.
Solid titanium is heated in oxygen gas to produce titanium dioxide.
It’s extremely dangerous to mix bleach and ammonia because highly toxic gas will form. So you should never mix these two chemicals. The two chemical reactions below describe what would happen if bleach and ammonia were mixed. If these reactions were carried out by a professional chemist using proper safety precautions in a controlled environment, what might that chemist observe in each case?
NaOCl(aq) + NH4OH(aq) → NH2Cl(g) + NaOH(aq) + H2O(l)
NaOCl(aq) + 2NH4OH(aq) → N2H4(g) + NaCl(aq) + 3H2O(l)
Look up chloramine, NH2Cl, and hydrazine, N2H4, on the Internet. Describe the toxicity of each compound.