LESSON 72: Atom Inventory: Balancing Chemical Equations
371
THINK ABOUT IT
INDUSTRY CONNECTION
INDUSTRY
CONNECTION
Ammonia can be made by first converting methane into another carbon compound and hydrogen gas. Then an iron compound is used as a catalyst to react hydrogen with nitrogen. Ammonia produced through this process is used to fertilize approximately one-third of the agricultural crops in the world.

The law of conservation of mass states that mass is not lost or gained in a chemical reaction. When you write a chemical equation to describe change, it is important that the equation follows this law. So any equation describing a chemical change must account for every atom involved.
How do you balance atoms in a chemical equation?
To answer this question, you will explore
Balancing Chemical Equations
Coefficients Are Counting Units
Balancing Chemical Equations
EXPLORING THE TOPIC
Balancing Chemical Equations
Imagine you’re in business to make ammonia, NH3, for farmers to fertilize their crops. It would be helpful to know how much nitrogen, N2(g), and hydrogen, H2(g), to combine so that nothing is wasted. How do you make sure you have the correct amount of nitrogen and hydrogen so that you have enough of each with no extra?
A chemical equation represents the exact ratio in which reactants combine to form products. A chemical equation that accounts for all the atoms involved and shows them combining in the correct ratio is called a balanced chemical equation. Learning how to balance chemical equations is a necessary part of working with chemical reactions.
FORMATION OF AMMONIA
The unbalanced equation below describes how nitrogen gas and hydrogen gas react to make ammonia gas.
To balance this equation, first take an inventory of the atoms on each side.
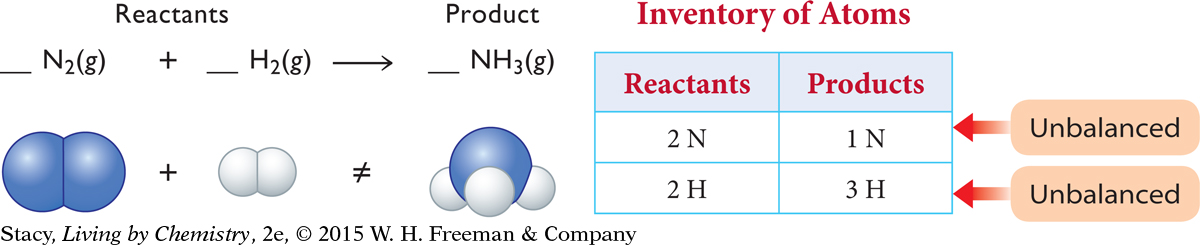
Next, balance the atoms on each side by adding units of N2, H2, or NH3. The product side needs one more N atom. The only way to accomplish this is to add a whole ammonia molecule, NH3, to the product side.
372
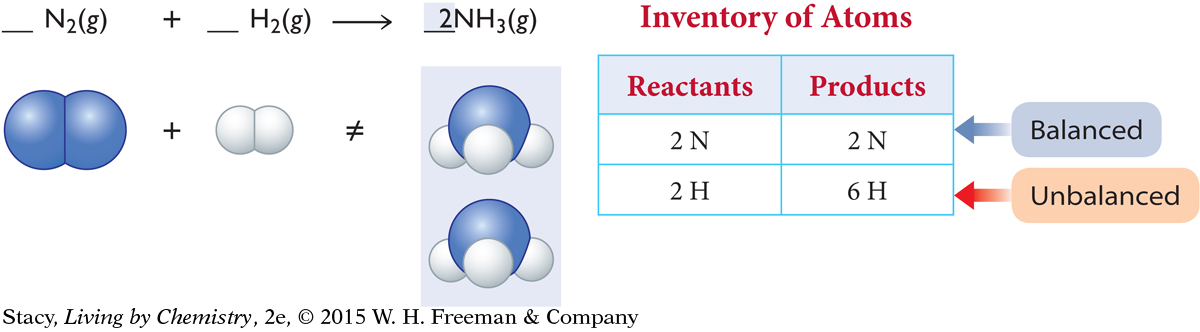
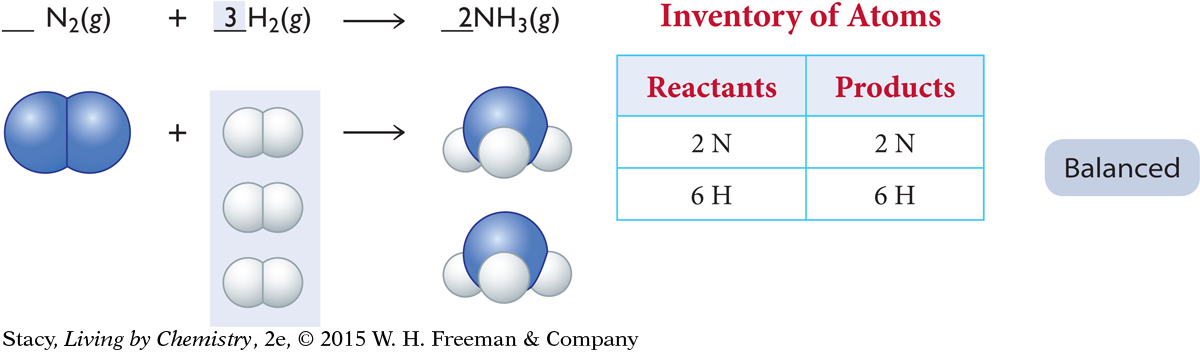
Take a new inventory. The N atoms are now balanced. Now the reactant side needs four more H atoms. The only way to accomplish this is to add two more molecules of H2 to the reactant side.
Important to Know
To balance a chemical equation, you can change only the coefficients, the numbers in front of each chemical formula. You cannot change the chemical formulas.
The equation is now balanced. The balanced equation shows that mass is conserved. There are the same numbers of N and H atoms on each side of the arrow.
In the balanced equation above, the 3 in front of the H2 and the 2 in front of the NH3 are called coefficients. Coefficients indicate how many units of each substance take part in the reaction. If there is no number in front of a chemical formula in an equation, the coefficient is understood to be a 1.
FORMATION OF RUST
Iron reacts with oxygen in the air to form iron (III) oxide, or rust. Iron (III) oxide is not a molecule. It is a highly organized collection of Fe and O atoms that are bonded to each other ionically. It is an ionic solid. There are a huge number of atoms in any piece of iron (III) oxide. But each piece will have two Fe atoms for every three O atoms. So Fe2O3 is the formula unit of iron oxide.
The unbalanced equation below describes how iron reacts with the oxygen in the air to form iron (III) oxide.
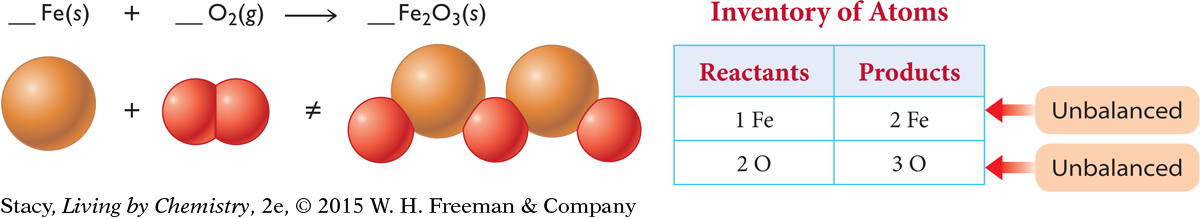
Balance the atoms on each side by adding molecules of O2, atoms of Fe, or formula units of Fe2O3. If you add an Fe atom to the reactant side, the Fe atoms are balanced. However, the O atoms are still not equal.
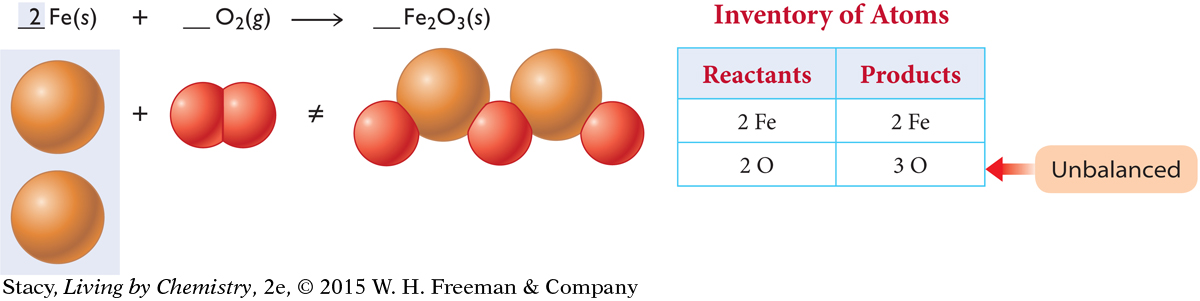
If you add two O2 molecules to the reactant side and one Fe2O3 formula unit to the product side, the oxygen atoms are balanced.
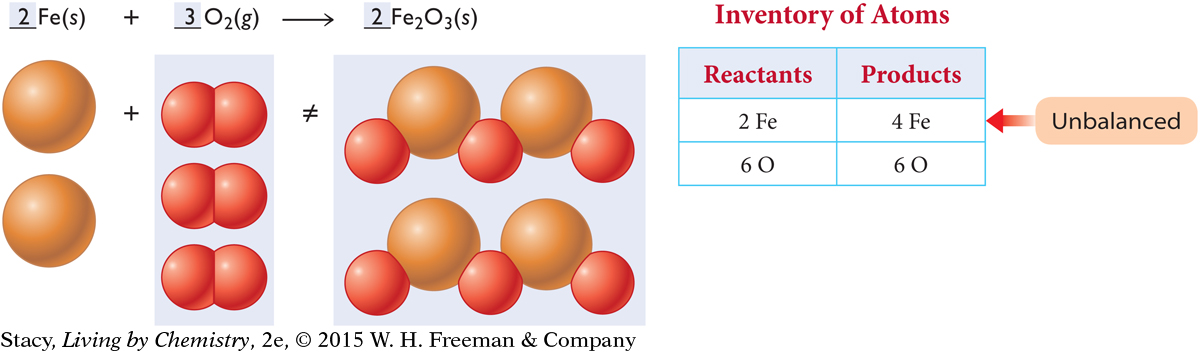
But now the iron atoms are unbalanced again. Add two more iron atoms on the reactant side, increasing the total to four. The balanced chemical reaction is shown here.
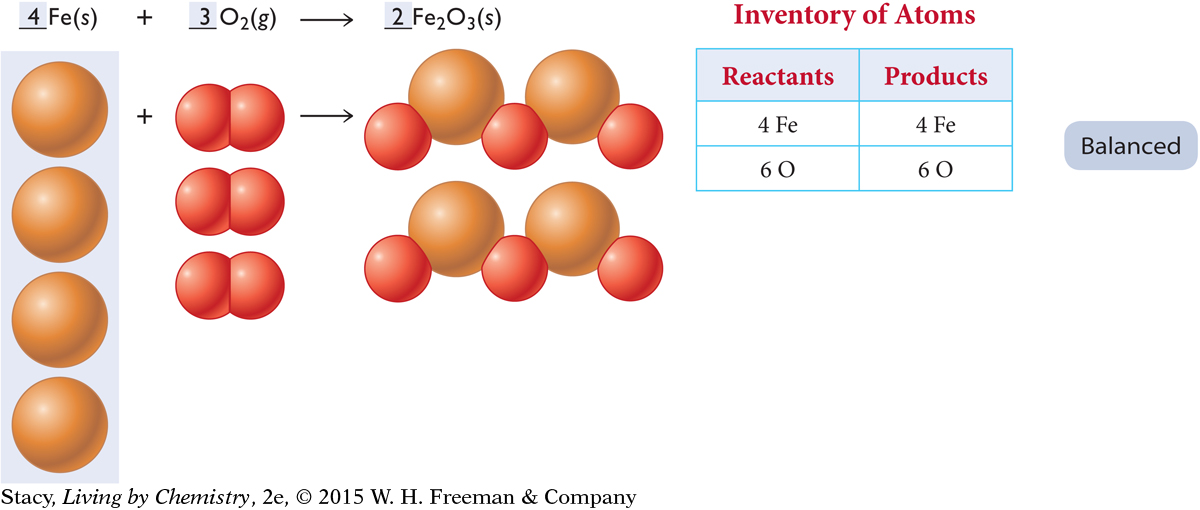
It took a few steps, but the equation is finally balanced. Four iron atoms combine with three oxygen molecules to form two formula units of iron oxide.
Coefficients Are Counting Units
Coefficients Are Counting Units
Once you have a balanced equation, multiplying all the coefficients by any counting unit will also give a balanced equation. You can multiply the coefficients by a dozen, or a thousand, or a million.
374
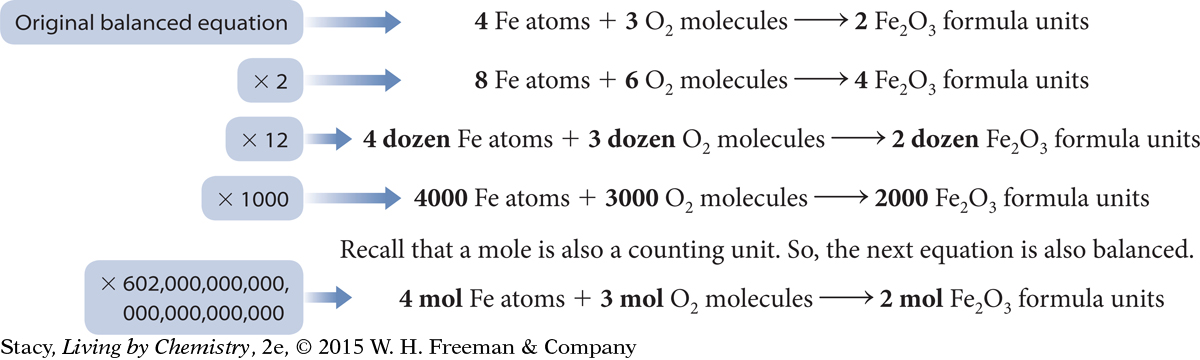
The coefficients in chemical equations can be multiples of any counting unit. The counting unit that chemists use most often is the mole. We can use moles to count the number of atoms, molecules, or formula units involved in a reaction.
LESSON SUMMARY
LESSON SUMMARY
How do you balance atoms in a chemical equation?
KEY TERMS
coefficient
formula unit
To conserve mass, the number of atoms on both sides of a chemical equation must be equal. When an equation is balanced, it shows how many molecules, atoms, or formula units of an ionic compound take part in a reaction and how many are produced. You can balance a chemical equation by working with the coefficients in front of the chemical formulas. The coefficients can be any counting unit, including moles. Units of mass or volume cannot be used as coefficients in chemical equations.
Exercises
Reading Questions
Why do chemical equations need to be balanced?
How are the coefficients in chemical equations different from the subscripts in chemical formulas?
Reason and Apply
Your recipe for banana bread calls for two ripe bananas. However, you have six ripe bananas that you want to use before they go bad.
How can you make banana bread with all six bananas?
How is this related to balancing a chemical equation?
Copy and balance these chemical equations.
K(s) + → I2(s) → KI(s)
Mg(s) + Br2(l) → MgBr2(s)
KBr(aq) + AgNO3(aq) → KNO3(aq) + AgBr(s)
KClO3(s) → KCl(s) + O2(g)
C2H6(g) + O2(g) → CO2(g) + H2O(l)
Al(s) + O2(g) → Al2O3(s)
P4(s) + H2(g) → PH3(g)