LESSON 73: What’s Your Reaction?: Types of Reactions
375
THINK ABOUT IT
Toxins work by reacting with chemicals in the body. These toxic reactions can remove compounds that are important to body function, or they can create new compounds that interfere with normal body processes. Classifying toxic reactions according to the different ways they react can help us to understand how toxic substances work in the body. It can also help us to come up with suitable approaches for dealing with them.
How do atoms rearrange to form new products?
To answer this question, you will explore
Combination and Decomposition Reactions
Exchange Reactions
Combination and Decomposition Reactions
EXPLORING THE TOPIC
Combination and Decomposition Reactions
Imagine that you are an ocean researcher and you spend your days underwater in a submarine. As you breathe, you use up oxygen gas, O2(g), and release carbon dioxide gas, CO2(g). Eventually, the high level of carbon dioxide and low level of oxygen in your submarine will be dangerous to your health. How do you remove carbon dioxide and produce oxygen gas to sustain life in a submarine?
COMBINATION REACTIONS
Combination reactions are used in air scrubbers on submarines to remove CO2(g) from the air. In a combination reaction, two reactants combine to form a single product. In Reactions 1 and 2, carbon dioxide combines with another compound to form a new product.
Reaction 1: Carbon dioxide gas reacts with solid sodium oxide to produce sodium carbonate (washing soda):
CO2(g) + Na2O(s) → Na2CO3(s)
Reaction 2: Carbon dioxide gas reacts with aqueous sodium hydroxide to produce sodium bicarbonate (baking soda):
CO2(g) + NaOH(aq) → NaHCO3(aq)
A combination reaction is sometimes represented by the general equation
A + B → C
DECOMPOSITION REACTIONS
In Reactions 3 and 4, compounds containing oxygen atoms decompose to produce O2(g) and an ionic solid. These decomposition reactions are two ways to produce oxygen. In a decomposition reaction, one reactant decomposes, or breaks down, to produce two or more products.
376
Reaction 3: Solid potassium chlorate decomposes to produce solid potassium chloride and oxygen gas:
2KClO3(s) → 2KCl(s) + 3O2(g)
Reaction 4: Solid barium peroxide decomposes to produce solid barium oxide and oxygen gas:
2BaO2(s) → 2BaO(s) + O2(g)
A decomposition reaction is sometimes represented by the general equation
A → B + C
On the International Space Station, oxygen is produced by a decomposition reaction that uses electrical energy to split water into hydrogen gas and oxygen gas:
2H2O(l) → 2H2(g) + O2(g)
Exchange Reactions
Exchange Reactions
Calcium is a necessary part of our diet, especially as we age and our bones become more fragile. An example of a calcium supplement is calcium carbonate, CaCO3(s). However, before the calcium in a vitamin tablet can become bone, it must be made more transportable in the bloodstream. It begins its chemical journey in the stomach.
ENGINEERING CONNECTION
ENGINEERING
CONNECTION
Inside submarines, air quality is a major concern. Not only must carbon dioxide be scrubbed out of the air but oxygen must be replenished and water vapor removed. Otherwise, the inside of the submarine becomes too damp from all the exhaled water vapor. For this reason, the air in a submarine is also pumped through dehumidifiers.

Examine the reactions involving calcium below. What patterns do you notice in the ways the atoms in the reactants are rearranged to produce the products?
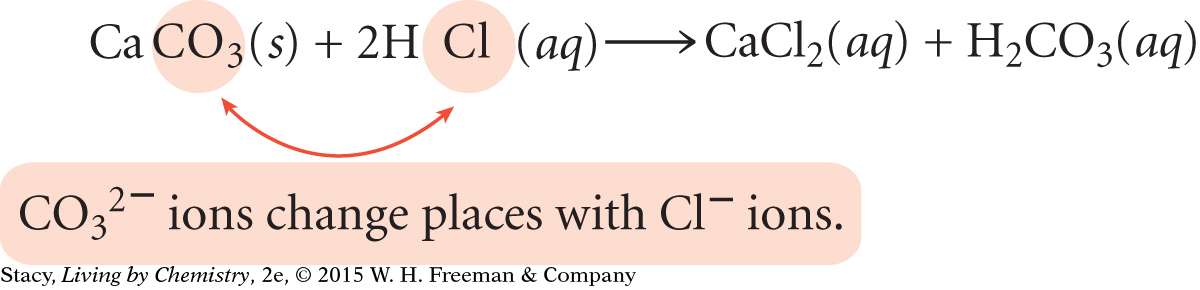
Reaction 5: Solid calcium reacts with hydrochloric acid in the stomach to produce aqueous calcium chloride and hydrogen gas:
Ca(s) + 2HCl(aq) → CaCl2(aq) + H2(g)
Reaction 6: Solid calcium carbonate reacts with hydrochloric acid (stomach acid) to produce aqueous calcium chloride and carbonic acid:
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2CO3(aq)
In Reaction 5, calcium atoms replace the hydrogen atoms in the HCl molecules.
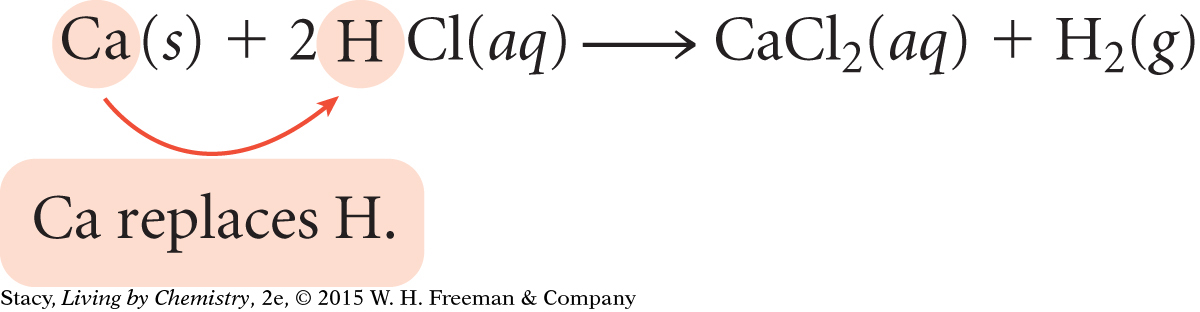
In Reaction 6, the polyatomic ion, CO32–, switches places with the chlorine ion, Cl–. The second reaction is a common way in which calcium is made available to your body through chemical changes that take place in your stomach.
377
In both cases, the two reactants exchange atoms. The first reaction is called a single exchange reaction or a single replacement reaction because atoms of an element are exchanged with atoms of another element in a compound. A single exchange reaction is sometimes represented by the general equation
A + BC → AC + B
The second reaction is called a double exchange reaction or a double replacement reaction because two compounds exchange atoms with each other. A double exchange reaction is sometimes represented by the general equation
AB + CD → AD + CB
Example
Classify Chemical Reactions
Classify each reaction as a combination reaction, a decomposition reaction, a single exchange reaction, or a double exchange reaction.
H2(g) + Cl2(g) → 2HCl(g)
CaCO3(s) → CaO(s) + CO2(g)
Sn(s) + O2(g) → SnO2(s)
CaI2(s) + Cl2(g) → CaCl2(s) + I2(g)
AgNO3(aq) + NaOH(aq) → AgOH(s) + NaNO3(aq)
Solution
Two reactants combine to produce one product. This is a combination reaction.
One reactant decomposes to produce two products. This is a decomposition reaction.
Two reactants combine to produce one product. This is a combination reaction.
Chlorine, Cl, replaces iodine, I, in CaI2. This is a single exchange reaction.
Silver, Ag, and sodium, Na, exchange places. This is a double exchange reaction.
Toxins sometimes act in the body in an exchange reaction by replacing an atom or atoms from a compound that exists in the body. This can have the effect of destroying necessary compounds or creating a new compound with harmful properties, or both. One way to remove toxic substances from the environment is by using combination and decomposition reactions.
LESSON SUMMARY
LESSON SUMMARY
How do atoms rearrange to form new products?
KEY TERMS
combination reaction
decomposition reaction
single exchange reaction (single replacement)
double exchange reaction (double replacement)
Chemical reactions can be classified into general categories based on how atoms in the reactants rearrange to form the products. Four general types of chemical change include combination reactions, decomposition reactions, single exchange reactions, and double exchange reactions. Toxins sometimes harm the body through chemical reactions. However, chemical reactions can also be used to remove toxins from the body or the environment.
378
Exercises
Reading Questions
How are combination reactions and decomposition reactions related?
What is the difference between a single exchange reaction and a double exchange reaction?
Reason and Apply
Classify these reactions as combination, decomposition, single exchange, or double exchange. Copy the equations, fill in any missing products, and write a balanced equation for each reaction.
NaOH(aq) + HNO3(aq) → NaNO3(aq) + ________(l)
C2H4(g) + Cl2(g) → ________(g)
Cl2(g) + MgBr2(s) → Br2(s) + ________(s)
List four molecules and four ionic compounds in the reactions from Exercise 3.
Sulfur trioxide gas combines with liquid water to produce aqueous sulfuric acid:
___________ (g) + H2O(l) → H2SO4(aq)
Predict the missing reactant, balance the equation, and explain how these toxic substances could enter the body.
Solid lithium reacts with aqueous hydrochloric acid to produce hydrogen gas and aqueous lithium chloride:
Li(s) + HCl(aq) → H2(g) + ___________
Predict the missing product, balance the equation, and explain how these toxic substances could enter the body.
Aqueous silver nitrate and aqueous sodium hydroxide are mixed to produce aqueous sodium nitrate and solid silver hydroxide:
AgNO3(aq) + NaOH(aq) → NaNO3(aq) + __________
Predict the missing product, balance the equation, and explain how the reaction could help remove toxic substances from water.