LESSON 76: Billions and Billions: Avogadro’s Number
390
THINK ABOUT IT
Mercury is a toxic substance that accumulates in the body and damages the central nervous system and other organs. Which do you think would be worse for you, 1 mole of mercury or 10 grams of mercury? To answer this question, it is necessary to understand the relationship between these two measures.
What is the relationship between mass and moles?
To answer this question, you will explore
Moles and Grams
Scientific Notation
Moles and Grams
EXPLORING THE TOPIC
Moles and Grams

In the world around you, many different measures are used to specify amounts. For example, at the store you might buy 10 apples or a 5 lb bag of apples. One measure is a counting unit and the other is a unit of weight. To compare the prices, you would probably want to know how many apples are in the 5 lb bag.
Likewise, chemists use both number and mass to specify the amount of a substance. For example, they might know the effect of either 1 mol of mercury atoms or of a 10 g sample of mercury. To compare these two quantities, they would need to know either how many moles of atoms are in each gram of mercury or the mass in grams of one mole of mercury.
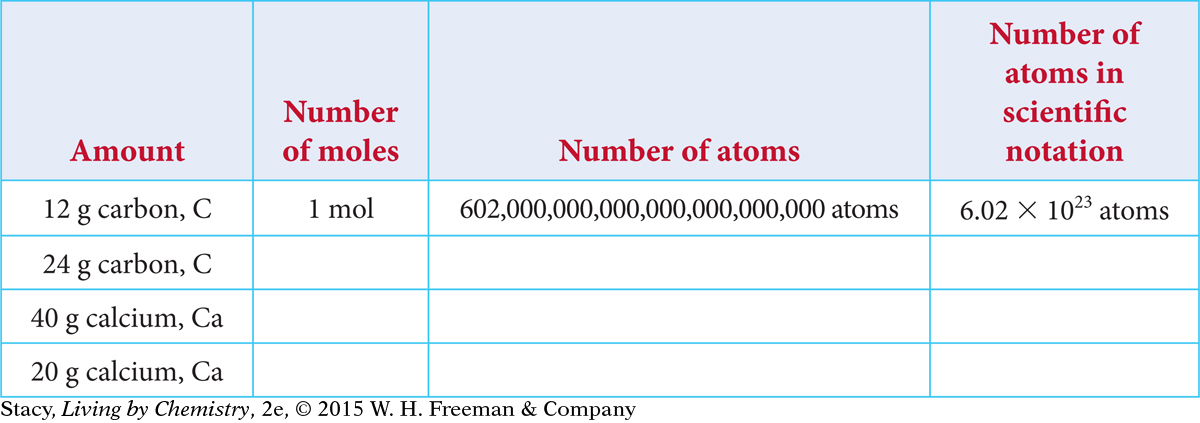
The mole, abbreviated mol, is a counting unit used to count a large number of atoms. A mole of atoms is equal to 602 sextillion atoms. This number is also referred to as Avogadro’s number.
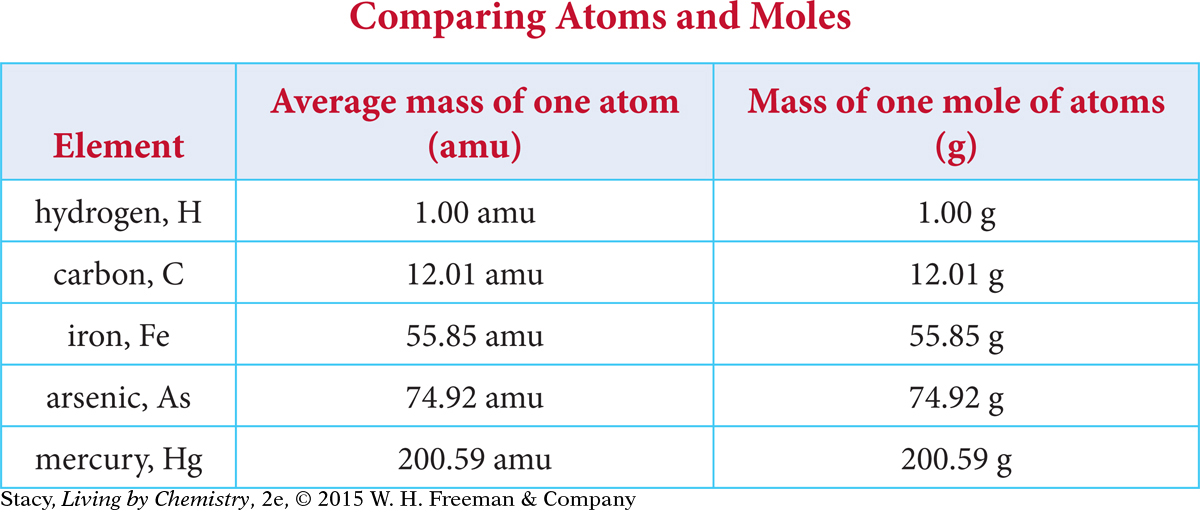
The table compares the average mass of one atom with the mass of 1 mol of atoms for five elements. Examine the table.
391
Notice that the numerical value on the periodic table for the average atomic mass of an element is identical to the mass of one mole of atoms of that element. In other words, both values are represented by the same number. What is different about the two measurements is the units. The average mass of one atom of arsenic is 74.92 amu, and the mass of one mole of arsenic atoms is 74.92 grams. How convenient!
Big Idea
Big Idea
The mass of one mole of atoms of an element in grams is numerically the same as the average atomic mass of that element.
MOLAR MASS
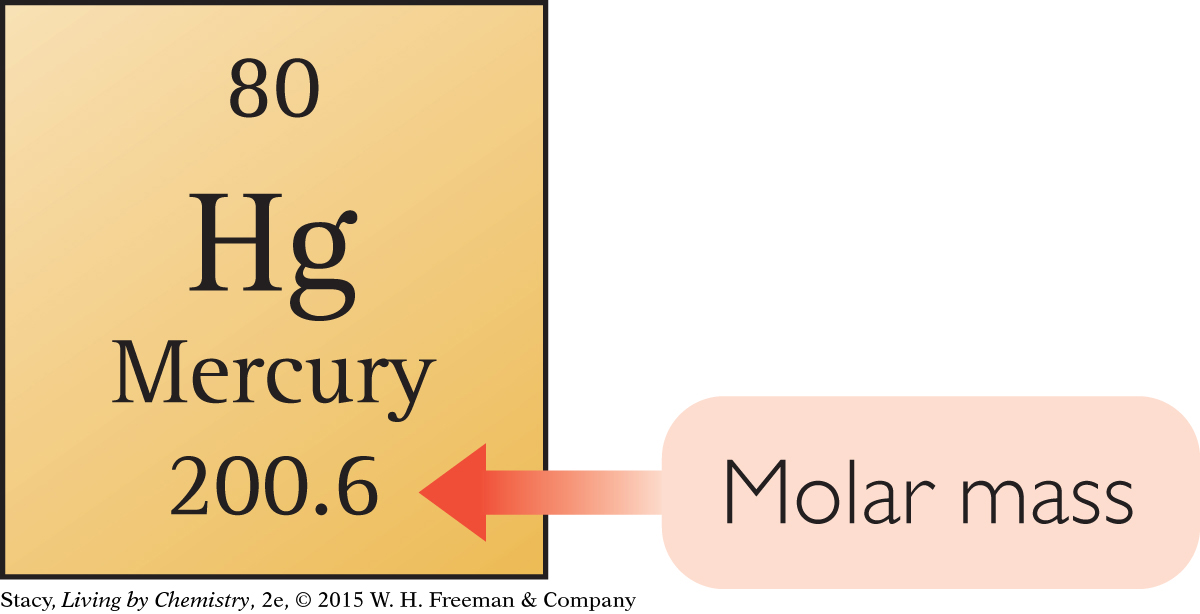
The mass of one mole of atoms of an element is called the molar mass. You can find the molar mass of any element by looking up the average atomic mass on the periodic table. For example, the average atomic mass of mercury is 200.6 amu. The mass of 1 mol of mercury atoms is 200.6 g.
Example 1
Copper versus Arsenic
Which has more mass, 1 mol of copper atoms or 1 mol of arsenic atoms?
Solution
Consult the periodic table to find the molar mass of each substance. The molar mass of copper is 63.55 grams per mole, and the molar mass of arsenic is 74.92 grams per mole. A mole of arsenic atoms has more mass.
Example 2
Amount of Mercury, Hg
Which is more toxic, 1 mol or 10 g of mercury, Hg?
Solution
By looking on the periodic table, you can determine that the mass in grams of 1 mol of mercury is 200.6 g. So, 1 mol of Hg is more toxic than 10 g of Hg because 1 mol represents a much larger mass.
Scientific Notation
Scientific Notation
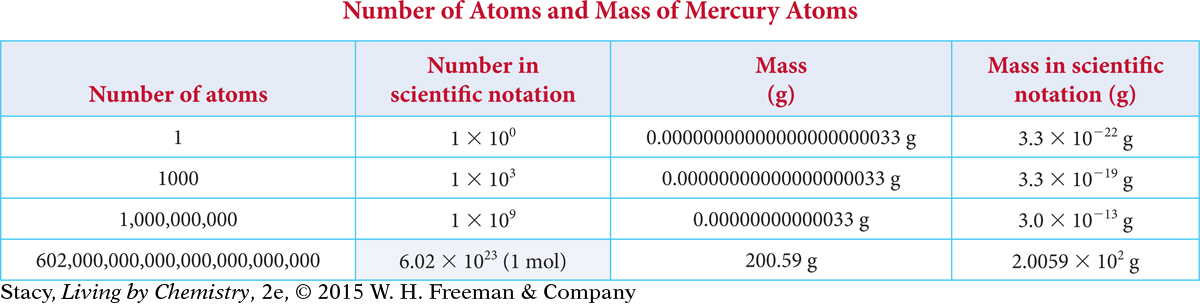
Consider the table on the next page. It shows the mass, in grams, of different numbers of atoms of mercury. Notice that the number of atoms and the mass are shown both in longhand and in scientific notation. Scientific notation is a shorthand method that uses an exponent to keep track of where the decimal point should be. Small numbers, between 0 and 1, have negative exponents. Numbers greater than 1 have positive exponents. The exponent indicates where the decimal point belongs in the longhand number.
392
ASTRONOMY CONNECTION
ASTRONOMY
CONNECTION
Each galaxy in the universe is estimated to have 400 billion stars in it. There are about 130 billion galaxies in the known universe. This is about 5.2 × 1022 total stars in the universe, or 0.08 mol.

In scientific notation, ten thousand is written 1.0 × 104. One ten-thousandth is written 1.0 × 10–4.
10,000 = 1.0 × 104
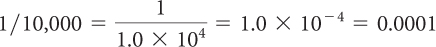
[For a review of this topic, see MATH Spotlight: Scientific Notation on page A-14.] The mass of 1000 atoms of arsenic is only 0.00000000000000000012 g or 1.2 × 10–19 g. This many arsenic atoms wouldn’t even be visible to the naked eye. On the other hand, 74.92 g of arsenic contains 1 mol, or 6.02 × 1023 atoms.
When you are dealing with atoms, numbers tend to be either very tiny or very large. So it is more convenient to use scientific notation.
LESSON SUMMARY
LESSON SUMMARY
What is the relationship between mass and moles?
KEY TERMS
molar mass
scientific notation
Chemists keep track of the number of molecules and atoms they are working with by using a unit called the mole. They also use scientific notation to express numbers that are very small or very large. One mole is equal to 602 sextillion. Using scientific notation, this number is written as 602,000,000,000,000,000,000,000, or 6.02 × 1023. The mass (in grams) of one mole of atoms of an element can be found on the periodic table. This is called the molar mass and has the same numerical value as the average atomic mass.
Exercises
Reading Questions
How do you find the average atomic mass of atoms of an element? What unit of measure is this given in?
What is meant by the term molar mass? What unit of measure is this given in?
Why do chemists convert between moles and grams?
Reason and Apply
393
Give the molar mass for the elements listed.
nitrogen, N
neon, Ne
chlorine, Cl
copper, Cu
Which has more mass?
1 mol of hydrogen, H, or 1 mol of carbon, C
1 mol of aluminum, Al, or 1 mol of iron, Fe
1 mol of copper, Cu, or 1 mol of gold, Au
5 mol of carbon, C, or 1 mol of gold, Au
Which contains more atoms?
12 g of hydrogen, H, or 12 g of carbon, C
27 g of aluminum, Al, or 27 g of iron, Fe
40 g of calcium, Ca, or 40 g of sodium, Na
40 g of calcium, Ca, or 60 g of zinc, Zn
10 g lithium, Li, or 100 g of lead, Pb
Which is more toxic? Explain your reasoning.
1 mol of beryllium, Be, or 10 g of beryllium
2 mol of arsenic, As, or 75 g of arsenic
2 mol of lead, Pb, or 500 g of lead
Copy and complete the table.