LESSON 78: Mountains into Molehills: Mass-Mole Conversions
398
THINK ABOUT IT
When you get a headache, you might reach for one of the many pain relievers on the market. A bottle of aspirin might direct you to take one 325 mg tablet. A bottle of acetaminophen might say to take one 500 mg tablet. Is one of these medications stronger than the other? One way to find out is to compare the number of moles of pain reliever in each dose.
How are moles and mass related?
To answer this question, you will explore
Relating Mass and Moles
Converting between Mass and Moles
Relating Mass and Moles
EXPLORING THE TOPIC
Relating Mass and Moles
You can find the mass of a substance by using a balance. However, you cannot directly measure the number of moles in a sample. You must convert the mass of a substance to moles to figure out how many atoms or molecules you have. If you want to compare amounts of two substances accurately, it is important to be able to convert between mass and moles.
EARTH SCIENCE CONNECTION
EARTH SCIENCE
CONNECTION
It is estimated that there are 2.0 × 1021 grains of sand on the entire Earth. This is only 0.003 mol of sand grains! In contrast, there is approximately 0.0002 mol of water molecules, or 1.2 × 1021 molecules, in a single drop of water.

Typically, you measure the mass of a substance in grams. Medications, like aspirin, are often measured in milligrams. Because there are 1000 milligrams in 1 gram, converting from milligrams to grams is simply a matter of dividing by 1000.
Consider three compounds commonly used in over-the-counter pain relievers.
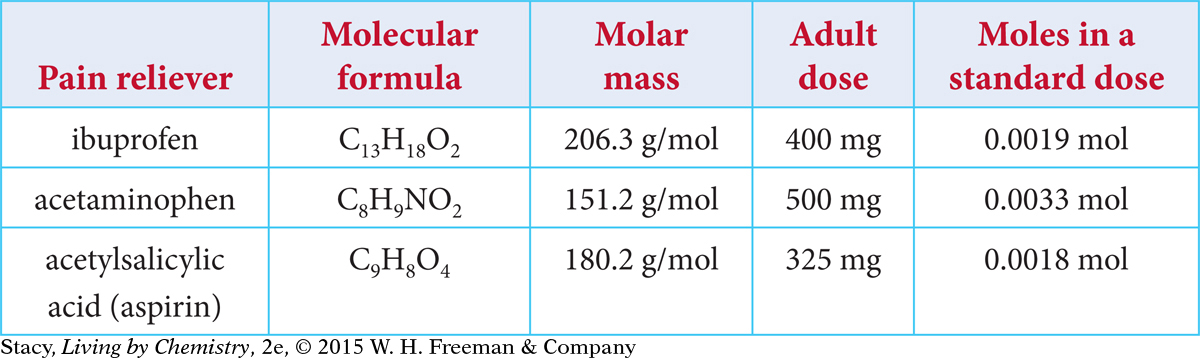
Notice that each pain reliever has a different adult dose. It would appear that acetylsalicylic acid (aspirin), with a smaller, 325 mg dose, is stronger than either acetaminophen, 500 mg, or ibuprofen, 400 mg. But ibuprofen is a heavier molecule than acetylsalicylic acid. One gram of aspirin will not represent the same number of molecules as one gram of ibuprofen. To make an accurate comparison, you must convert the milligrams of each compound to moles.
399
Examine the table. Which medication contains the most moles of pain reliever? A 500 mg dose of acetaminophen contains almost twice as many moles of pain reliever as a 325 mg dose of acetylsalicylic acid. So aspirin does appear to be more powerful than acetaminophen. Acetylsalicylic acid has the lowest dose and uses the fewest molecules of pain reliever to get rid of your headache. Ibuprofen is almost identical in potency to acetylsalicylic acid. According to the data, acetaminophen is not as powerful as the other two compounds.
Converting between Mass and Moles
Converting between Mass and Moles
The relationship between the mass in grams of a substance and moles is proportional. The proportionality constant is the molar mass, in g/mol. The molar mass will be different for each substance.
Mass-Mole Conversions The molar mass is the mass per mole of a substance. You can use it to convert between the mass, m, and the number of moles, n.
Example 1
Converting from Moles to Mass
Imagine that a pharmaceutical company has 100 mol of acetaminophen available to be made into 500 mg tablets. The molar mass of acetaminophen is 151.2 g/mol. How many 500 mg tablets can be made?
Solution
First, figure out how many grams of acetaminophen there are in 100 mol.
| Start with the mole to mass formula. | m = molar mass · n |
| Substitute values and solve for mass. | = (151.2 g/mol) 100 mol |
| = 15,120 g |
Because each 500 mg tablet is 0.5 g, each gram of the compound will make two tablets.
(15,120 g) (2 tablets/g) = 30,240 tablets
So 30,240 tablets can be made from 100 mol of acetaminophen.
Example 2
Converting from Mass to Moles
Restaurants usually provide small packets of sugar to sweeten coffee or tea. Each sugar packet contains approximately 1.0 g of sucrose, C12H22O11. How many moles of sucrose does this represent?
Solution
400
First, use the periodic table to determine the molar mass of sucrose. The molar mass of sucrose is 342.3 g/mol rounded to one decimal place. To convert from mass to moles, divide the mass in grams by the molar mass.
| Rearrange the mole to mass formula to solve for n. | 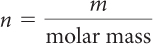
|
| Substitute values and solve for moles of sucrose. | 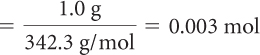
|
So one sugar packet contains 0.003 mol of sucrose.
LESSON SUMMARY
LESSON SUMMARY
How are moles and mass related?
Molar mass values allow you to convert between mass in grams of a substance and moles. To convert mass to moles, divide the mass of a sample in grams by its molar mass. To convert moles to mass, multiply the number of moles of the substance by its molar mass.
Exercises
Reading Questions
How can you convert between moles of a substance and grams of a substance?
Why might a 200 mg tablet of aspirin not have the same effect as a 200 mg tablet of ibuprofen?
Reason and Apply
There are 8.0 mol of H atoms in 2.0 mol of CH4O molecules. How many moles of H atoms are there in 2.0 mol of C2H6O molecules?
List these compounds in order of increasing moles of molecules: 2.0 g CH4O, 2.0 g H2O, 2.0 g C8H18. Show your work.
Which has more moles of oxygen atoms, 153 g of BaO, or 169 g of BaO2? Show your work.
List these compounds in order of increasing mass in grams: 2.0 mol SiCl4, 2.0 mol PbO, 2.0 mol Fe2O3. Show your calculations.
Copper is the third most used metal after iron and aluminum. Copper usage is rapidly expanding as more products are developed that contain electronic components. Suppose you run a company that buys copper compounds and then recycles the copper for resale. Your company wants to get the most pure copper for the lowest cost. Three different suppliers want to sell you 1 mol CuO(s), 1 mol CuCO3(s), and 1 mol Cu2O(s) for the same price.
Which compound has the greatest total mass? Show your work.
Which compound has the greatest mass of Cu? Show your work.
Assuming it costs the same to extract the copper from each compound, which compound represents the best deal for your company? Explain.
401
Suppose Container A contains 1 mol C(s) and 1 mol O2(g), and Container B contains 1 mol CO2(g). Both containers are closed and both are the same size. Compare the containers in each of these ways.
number of atoms
number of gas molecules
mass
gas pressure