Featured LAB: Solution Concentration
Featured LAB
408
Solution Concentration
Purpose
To explore solutions and the concentration of dissolved solids in solution.
Part 1: Dissolving Solids
Materials
sugar, C12H22O11, 60 g
salt, NaCl, 60 g
water, 200 mL
250 mL beakers (2)
balance
stirring rod
2 spatulas or plastic spoons
2 green or red gummy bears
Procedure
Make predictions. How many grams of sugar do you think you can dissolve in 100 mL of water? (1 tsp = 4 g) How many grams of salt? (1 tsp = 6 g)
Determine how many grams of sugar will dissolve in 100 mL of water at room temperature. Keep adding sugar and stirring until you notice that the beaker contains solid sugar that will not dissolve.
Repeat this process using table salt, NaCl, instead of sugar.
Calculations
Compare the number of grams of dissolved solid per liter of solution.
Compare the number of moles of solid per liter of solution.
Explain why the sugar solution has the greater mass of dissolved solid per liter but the salt solution has the greater number of moles of solid per liter.
Part 2: Gummy Bears
Five gummy bears were placed overnight in aqueous sugar solutions as shown.
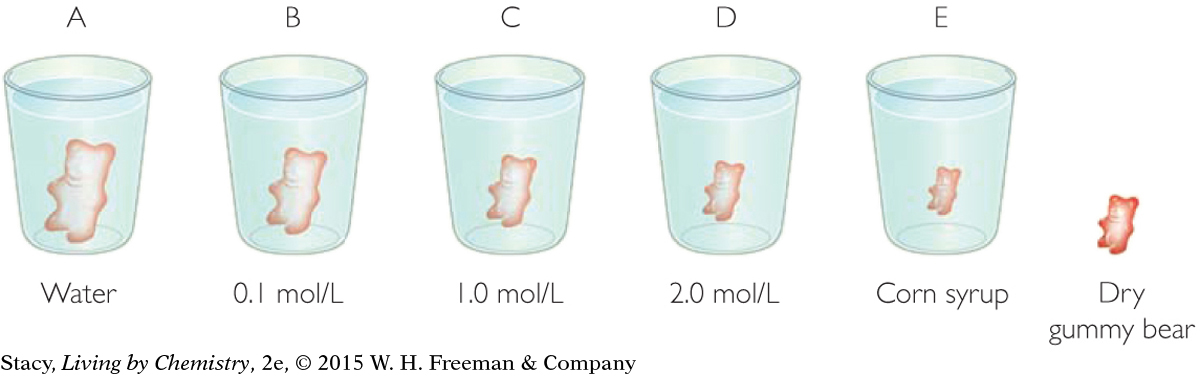
Describe what happens to the bears in the solutions. Which solution had the greatest effect on the gummy bears? Why do you think this is so?
What evidence do you have that it is water and not sugar that is moving into and out of the bears?
Place a gummy bear in each saturated solution you created. Predict what they will look like after 24 hours. Explain your reasoning.