LESSON 83: Is It Toxic?: Mystery Solutions
422
THINK ABOUT IT
Selling bottled water is a big business. Some companies claim to get their water from mountain streams. Some say that their water is quite pure. Still others claim their water contains beneficial minerals. How could you determine how pure a water sample is?
Can molarity calculations be used to identify a toxic substance?
To answer this question, you will explore
Accounting for the Mass of a Solution
Drinking Water Standards
Accounting for the Mass of a Solution
EXPLORING THE TOPIC
Accounting for the Mass of a Solution
Suppose that you want to know how pure a sample of water is. Examine the illustration and consider how you might use mass to make a determination of purity.
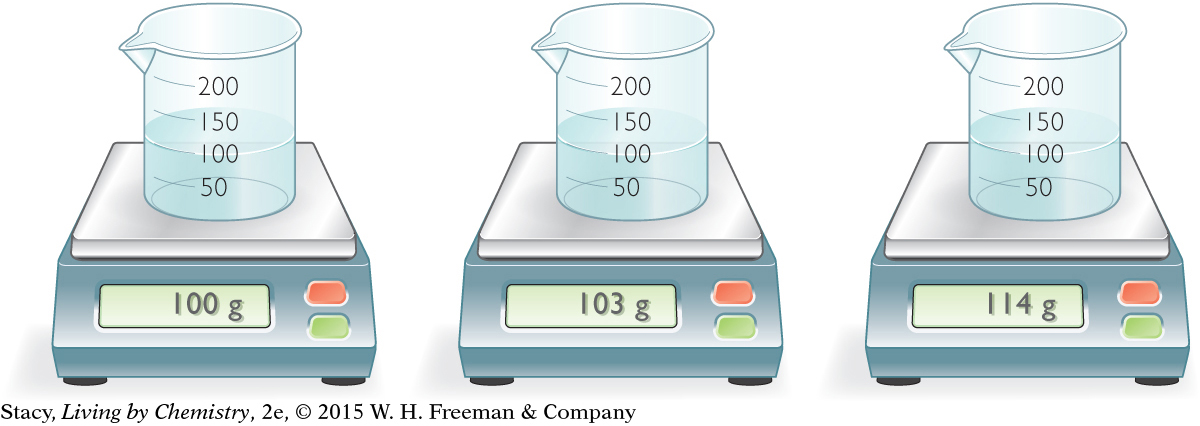
What masses do you expect if the samples are all pure water? Recall that mass per volume is equal to density. The density of pure water is 1 g/mL. If a 100 mL sample is pure water, then you expect a mass of 100 g.
The sample in the first beaker may be pure water. The other two samples must contain solutes that add more mass to the same volume, making them denser (and therefore heavier) than pure water.
THE DENSITY OF A SOLUTION
Suppose you have three samples of salt water with the concentrations shown in the illustration at the top of the next page. Take a moment to consider how the densities of the three solutions compare. Why does the ball float higher in the solution on the right?
423
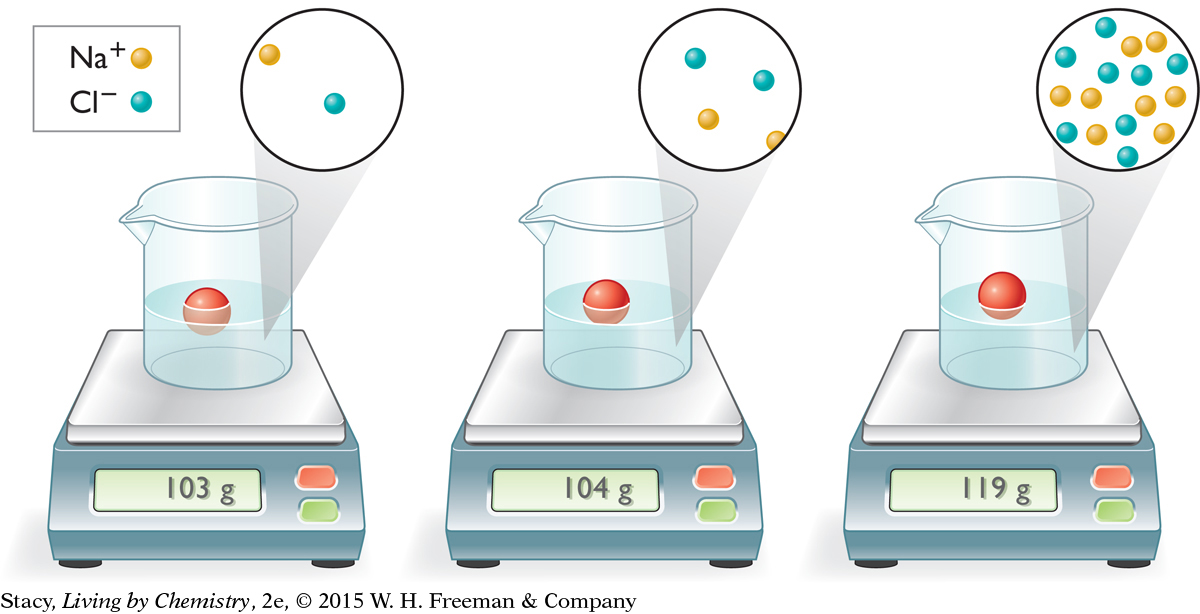

As the density of the solution increases, an object floats higher. That is why when you swim in very salty water, you float with more of your body out of the water. As the concentration increases, there are more units of NaCl per volume of solution. The higher the salt concentration, the denser the solution.
SAME MASS, DIFFERENT SOLUTES
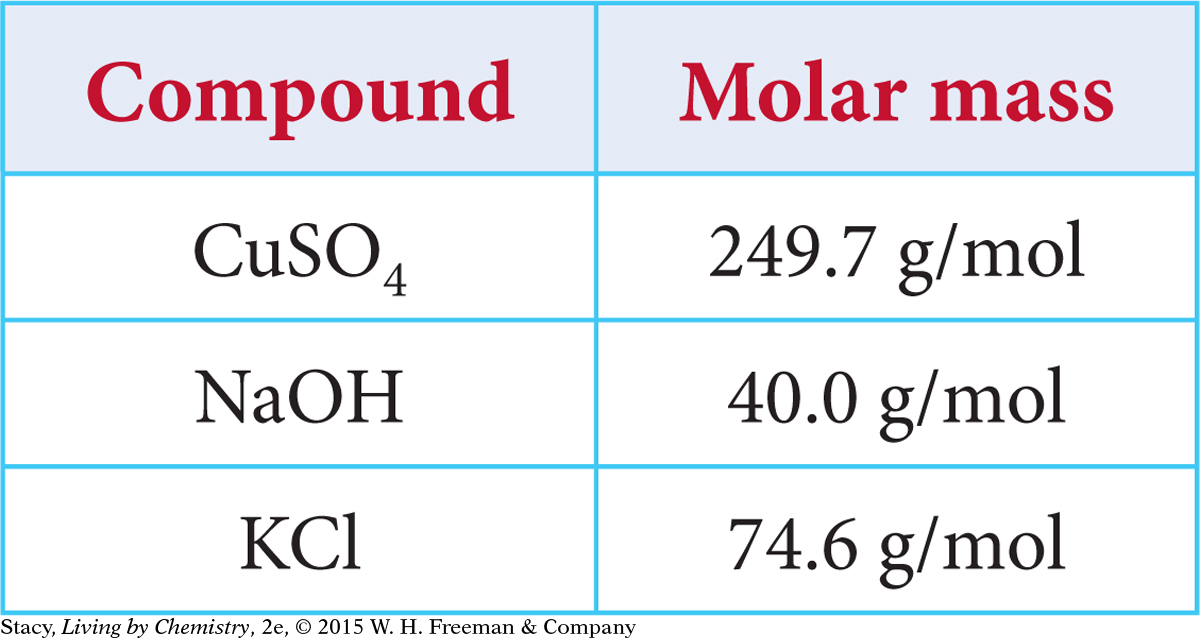
Imagine that you add 10 g of three different solids to three different beakers and then add enough water to make 100 mL of solution. The solutions all have approximately the same mass—the mass of about 100 mL of water plus 10 g of solute. But do they have the same concentration?
Copper (II) sulfate, CuSO4 has the largest molar mass. That means there are fewer formula units of CuSO4 in 10 grams compared with NaOH. While you’ve added the same mass to each sample, you have not added the same number of moles. Dissolving 10 grams of the compound with the higher molar mass will result in a lower concentration.
424
Example
Different Solutions, Same Concentration
Suppose you create 0.10 M solutions of copper (II) sulfate, sodium hydroxide, and potassium chloride. For equal volumes, which solution will have the most mass?
Solution
The mass of each solution is the mass of the water plus the mass of solute. The mass of the solute added depends on its molar mass. To determine the mass corresponding to 0.10 mol of each solute, multiply the number of moles by the molar mass of each compound.
Mass of CuSO4 = (249.7 g/mol)(0.10 mol) = 25.0 g CuSO4
Mass of NaOH = (40.0 g/mol)(0.10 mol) = 4.0 g NaOH
Mass of KCl = (74.6 g/mol)(0.10 mol) = 7.5 g KCl
So, for equal volumes of solution, the CuSO4(aq) will have the most mass.
Drinking Water Standards
Drinking Water Standards
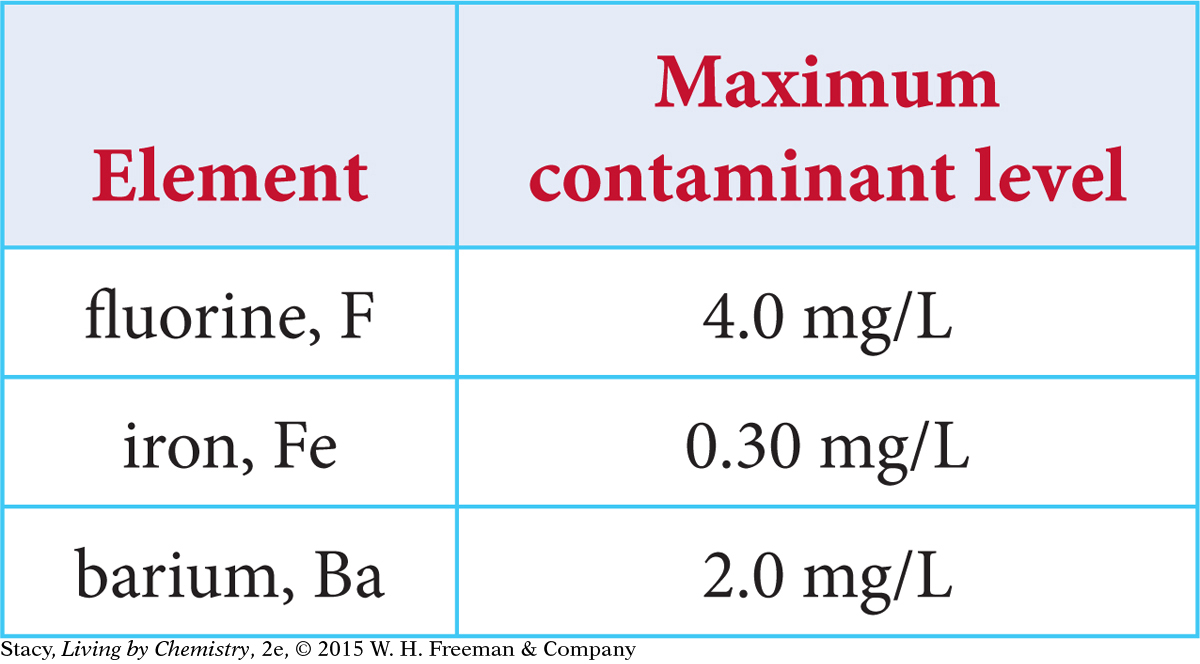
Environmental Protection Agency standards protect public health by limiting contaminants in drinking water. The maximum contaminant levels for fluorine, iron, and barium in drinking water are given in mg/L in the table.
ENVIRONMENTAL CONNECTION
ENVIRONMENTAL
CONNECTION
The Cuyahoga River in northeastern Ohio used to be so polluted that it caught fire several times between 1936 and 1969. In 1969, an article about the fires in Time magazine led to the Clean Water Act, the Great Lakes Water Quality Agreement, and the creation of the Environmental Protection Agency, or EPA.

Based on the numbers in the table, you might conclude that iron, Fe, is the most toxic because the allowable level is the smallest by mass. However, these contaminants interact with your body atom by atom. So you need to compare the contaminants by number of atoms.
Find the concentration of the potential toxins in moles per liter. To do so, convert the maximum contaminant level milligrams to grams, and then divide by the molar mass.
| fluorine, F | 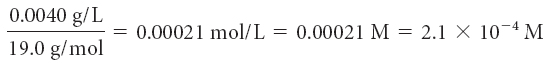
|
| iron, Fe | 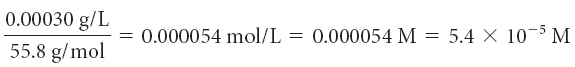
|
| barium, Ba | 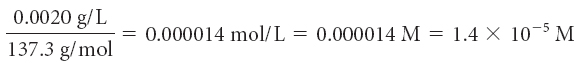
|
In moles per liter, the maximum contaminant level for barium, Ba, is the smallest numerical value. This means less of it is tolerated in our drinking water, making barium the most toxic of the three elements.
LESSON SUMMARY
LESSON SUMMARY
425
Can molarity calculations be used to identify a toxic substance?
When a substance is dissolved in water, it adds to the mass of the solution. One mole of one substance may have a very different molar mass than one mole of another substance. So different solutions of the same concentration can be differentiated by weighing them. Comparing contaminants by number of atoms may give a different picture of toxicity than comparing them by mass.
Exercises
Reading Questions
Explain how you might use mass to determine if a sample of water is contaminated.
Explain why the density of a solution increases as the concentration increases.
Explain why 0.10 M CuCl2 has a greater density, m/V, than 0.10 M KCl.
Reason and Apply
Find the molarity of each solution. Which solution has the highest molarity? The highest density? Give your reasoning.
1.0 g KCl in 1.0 L water
5.0 g KCl in 0.5 L water
10.0 g KCl in 0.3 L water
Find the molarity of each solution. Which solution has the highest molarity? The highest density? Give your reasoning.
10 g NaNO3 in 1.0 L water
10 g Cd(NO3)2 in 1.0 L water
10 g Pb(NO3)2 in 1.0 L water
Find the mass of solute in each solution. Which solution has the highest density? Is it possible to calculate the density of each solution? Why or why not?
1.0 L of 0.10 M NaNO3
1.0 L of M 0.10 M Cd(NO3)2
1.0 L of 0.10 M Pb(NO3)2
Which would weigh the most? Explain your thinking.
1.0 L of 1.0 M NaBr
500 mL of 1.0 M KCl
1.0 L of 0.5 M NaOH
Look up the MSDS or Materials Safety Data Sheet, for sodium chloride, NaCl, and sodium sulfate, Na2SO4. Describe the toxic effects of each salt.
What are the LD50’s (mouse, oral)?
Compare the toxicities based on weight in grams.
Compare the toxicities based on moles.
Compare the toxicities based on volume of 0.10 M solution.