LESSON 86: pHooey!: [H+] and pH
437
THINK ABOUT IT
During delivery, a baby can sometimes go into distress. So doctors monitor the vital signs to make sure the baby is okay. One test is called a fetal blood test. This test involves taking the pH of the baby’s blood. If the baby’s blood has a pH of 7.2 or lower, the doctors know that the baby is not getting enough oxygen and is in danger. A pH of around 7.3 indicates that the baby is safe.
How is pH related to the acid or base concentration of a solution?
To answer this question, you will explore
Acid Concentration
Relationship between [H+] and pH
pH of Water and Basic Solutions
Acid Concentration
EXPLORING THE TOPIC
Acid Concentration
If you are testing the acidity of a solution by determining pH, you are determining the hydrogen ion concentration in moles per liter. When a solution has a greater hydrogen ion concentration than water does, it is an acid. Chemists use a standard notation with brackets to symbolize concentration in moles per liter. For example, [H+] symbolizes the concentration of hydrogen ions, H+, in moles per liter.
PARTICLE VIEWS OF ACIDIC SOLUTIONS
To get an idea of how pH numbers relate to hydrogen ion concentration, it is useful to compare particle views of solutions. The illustration shows the ions in two hydrochloric acid solutions, one that is 0.010 M and the other 0.002 M. How is the hydrogen ion concentration, [H+], related to the pH?
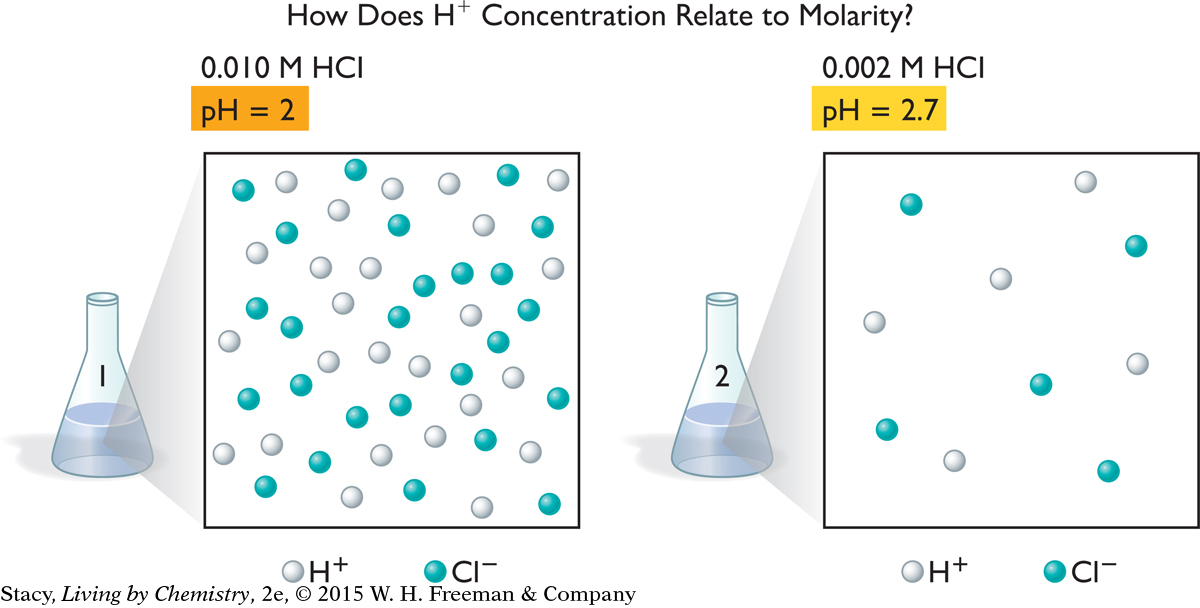
438
In the illustration, solution 1 has five times as many H+ cations and five times as many Cl− anions as solution 2. However, the difference in the pH is only 0.7. Small changes in pH signal large differences in hydrogen ion concentrations.
Big Idea
Big Idea
The greater the concentration of H+ ions in a solution, the lower its pH, and the more acidic it is.
Relationship between [H+] and pH
Relationship between [H+] and pH
The hydrogen ion concentration of a solution is directly related to the pH of that solution. The table shows the H+ concentration and the pH number for a few different concentrations of hydrochloric acid.
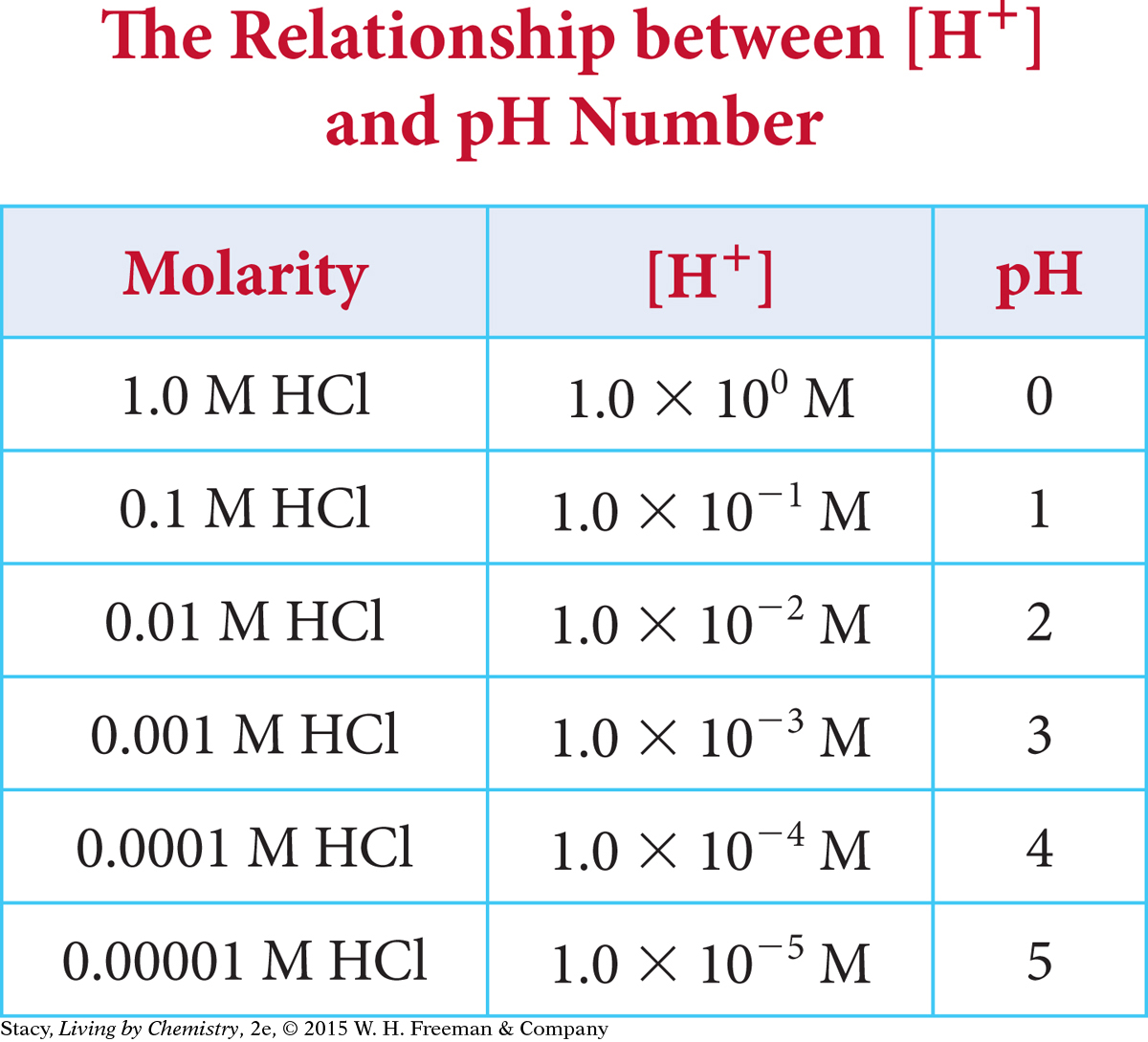
Notice that each time the H+ concentration changes by a factor of 10, the pH number changes by 1 unit. You can also see that the pH number is equal to the exponent, without the negative sign. For example, 1.0 × 10−4 M HCl has a pH of 4. This is true whenever the H+ concentration is expressed in scientific notation and the coefficient is 1.0.
Each unit of change in pH represents a tenfold difference in H+ concentration. This is called a logarithmic relationship. The mathematical formula connecting pH to [H+] is
pH = −log [H+]
You can use this formula and a calculator to convert between pH and [H+].
[For review of this math topic, see MATH Spotlight: Logarithms on page A-17.]
Example 1
Calculating pH for an HCl Solution
What is the pH of a 0.000001 M HCl solution?
What is the pH of a 4.2 × 10−3 M HCl solution?
439
Solution
Each HCl formula unit dissociates into an H+ and a Cl− ion. So the concentration of H+ ions is 0.000001 M, which can also be written 1.0 × 10−6 M. Because the coefficient is 1.0, the pH is the exponent without the minus sign. The pH is 6.
Use the relationship pH = −log [H+].
pH = −log [4.2 × 10−3] = 2.4
The pH of the 4.2 × 10−3 molar HCl solution is between 2 and 3. This makes sense because the H+ concentration is between 1.0 × 10−2 M and 1.0 × 10−3 M.
pH of Water and Basic Solutions
pH of Water and Basic Solutions
Basic solutions also have pH numbers associated with them. This is because they also have an H+ concentration and pH value.
The number line below shows the relationship between pH and H+ concentration for acidic, basic, and neutral solutions at 25 °C. The H+ concentration in basic solutions is quite small. For example, a solution with a pH of 14 has a hydrogen ion concentration of 1.0 × 10−14 M, or 0.00000000000001 M.
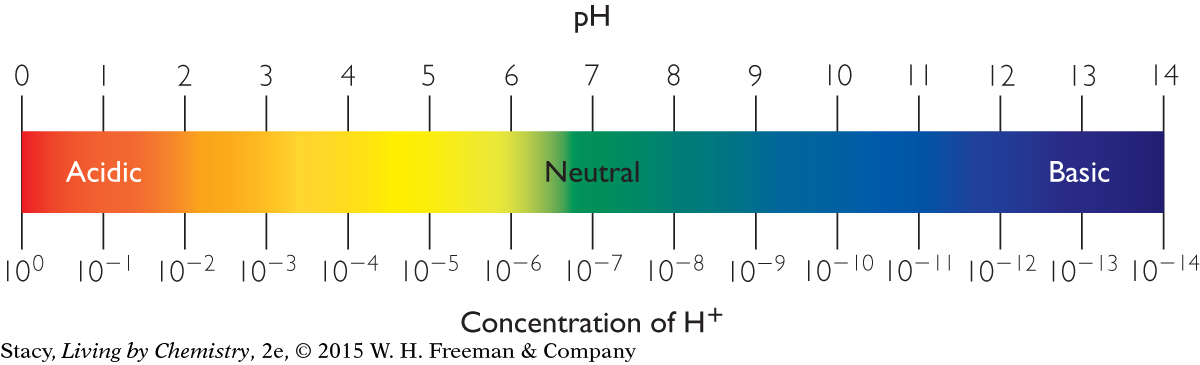
DISSOCIATION OF WATER
It may seem odd that a basic solution has hydrogen ions in it. To understand this, it is necessary to consider water on a molecular level.
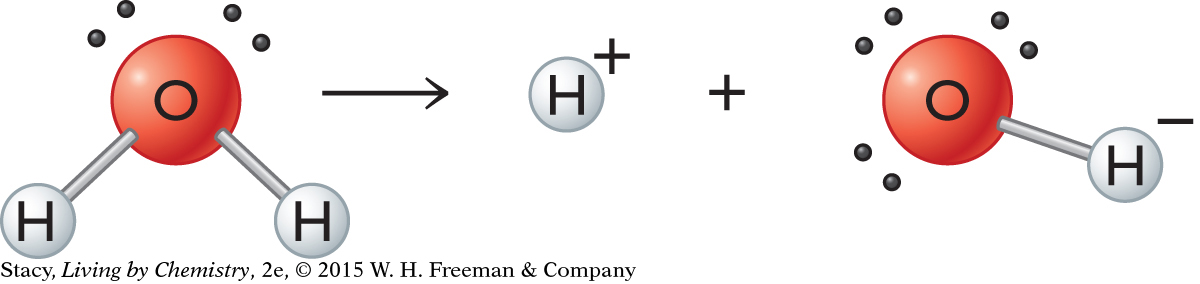
Notice that the hydrogen ion concentration in a neutral solution, such as water, is listed as 1.0 × 10−7 M H+ at 25 °C. Water molecules dissociate slightly, forming H+ and OH− ions. The dissociation is so slight that there are only 2 H+ ions and 2 OH− ions for every 1 billion water molecules. This translates to an H+ concentration of 1 × 10−7 M, or a pH of 7. Water is considered neutral because it contains equal amounts of H+ and OH−.
When a substance dissolves in water and the H+ concentration, [H+], remains equal to the OH− concentration, [OH−], we say that substance is neutral. For example, NaCl dissolves in water to form Na+ cations and Cl− anions. This does not change the balance of H+ and OH− ions already present in the water. NaCl(aq) is neutral.
440
When an acid such as HCl dissolves in water, it breaks apart into H+ and Cl− ions, increasing the ratio of H+ ions to OH− ions. Likewise, when a substance like NaOH dissolves in water, it breaks apart into Na+ and OH− ions. This also alters the balance of H+ and OH− ions in the water. In the case of NaOH(aq), the hydrogen ion concentration, [H+], is less than the hydroxide ion concentration, [OH−], so NaOH is considered a base.
The table shows the concentration of H+ and OH− ions in a variety of sodium hydroxide solutions.
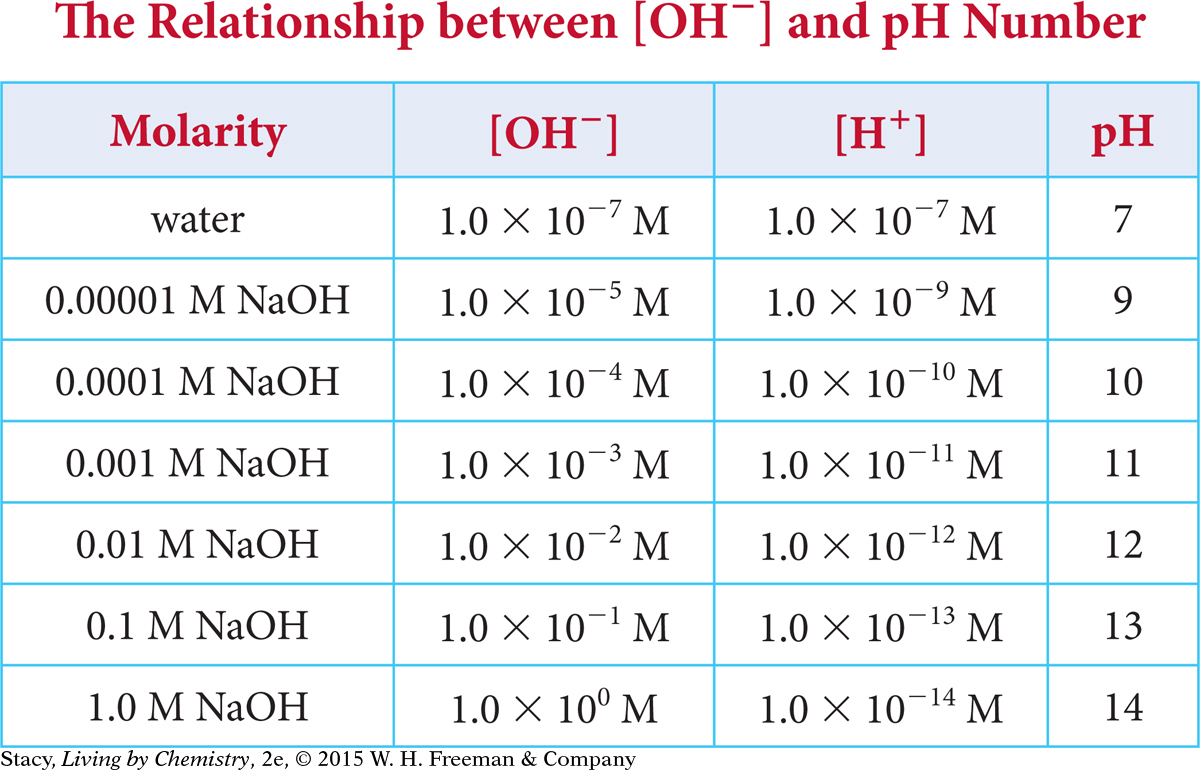
Notice that the H+ concentration decreases as the OH− concentration increases and vice versa. There is a mathematical relationship between [H+] and [OH−]:
[H+] [OH−] = 1 × 10−14
In any aqueous solution at 25°C, the hydrogen ion concentration multiplied by the hydroxide ion concentration is equal to 1.0 × 10−14. For example, when the OH− concentration is 1 × 10−3 M, the H+ concentration is 1 × 10−11 M.
(1 × 10−11)(1 × 10−3) = 1 × 10−14
You can also see that adding the values of the exponents always results in a total of −14.
Example 2
Calculating pH for a Solution of Sodium Hydroxide, NaOH
What is the pH of a 0.000001 M NaOH solution?
Solution
A solution with a molarity of 0.000001 M NaOH has an OH− concentration of 1.0 × 10−6 moles per liter. The H+ concentration must be 1.0 × 10−8 M because the exponents must sum to −14. The pH is just the exponent of the H+ concentration without the negative sign. So the pH is 8.
LESSON SUMMARY
LESSON SUMMARY
441
How is pH related to the acid or base concentration of a solution?
The pH number and the hydrogen ion concentration of a solution are related logarithmically: pH = −log [H+]. This means that when the hydrogen ion concentration changes by a factor of 10, the pH changes by 1. Water and basic solutions also have pH numbers because they contain small concentrations of H+ ions. Water dissociates slightly into H+ and OH− ions. In pure water, the H+ concentration is 1 × 10−7 M and the pH is 7. The hydroxide ion concentration and the hydrogen ion concentration in a solution are related mathematically: [H+][OH−] = 1.0 × 10−14.
Exercises
Reading Questions
What is the relationship between pH and H+ concentration?
How is the H+ concentration related to the OH− concentration in a solution?
Reason and Apply
What pH would you expect for solutions with the concentrations given here?
[H+] = 1.0 × 10−4 M
[H+] = 1.0 × 10−12 M
[OH−] = 1.0 × 10−8 M
Determine the pH for solutions with the H+ concentrations given here.
[H+] = 0.0014
[H+] = 6.0 × 10−8 M
[H+] = 4.2 × 10−11 M
[H+] = 1.5 × 10−1 M
Which of the solutions in Exercise 4 are acids? Which are bases?
What H+ concentration would you expect for the solutions below?
pH = 9
pH = 7.3
pH = 2.9
pH = 10.2
What is the pH of a 2.5 M HCl solution?
What is the pH of a 0.256 M NaOH solution?
The Richter scale is a logarithmic scale for measuring the magnitude of earthquakes. How much more intense is a Richter 7.0 earthquake than a Richter 5.0 earthquake?