LESSON 89: Drip Drop: Titration
451
THINK ABOUT IT
When sulfur dioxide, SO2, and nitrogen oxide, NO, two components of air pollution, come in contact with water in the atmosphere, they are converted to sulfuric acid, H2SO4, and nitric acid, HNO3. The acids then fall as acid rain or snow. Acidic precipitation is highly destructive, sometimes causing the complete elimination of fish and insect species from lakes or streams. Acid rain also makes the soil unsuitable for plant life, killing off whole sections of forest. Water scientists, called hydrologists, regularly study the acidity of lakes and streams to monitor the extent of the acid rain problem.
How can you use a neutralization reaction to figure out acid or base concentration?
To answer this question, you will explore
Titrations
Particle Views of Titrations
Titration Calculations
Titrations
EXPLORING THE TOPIC
Titrations
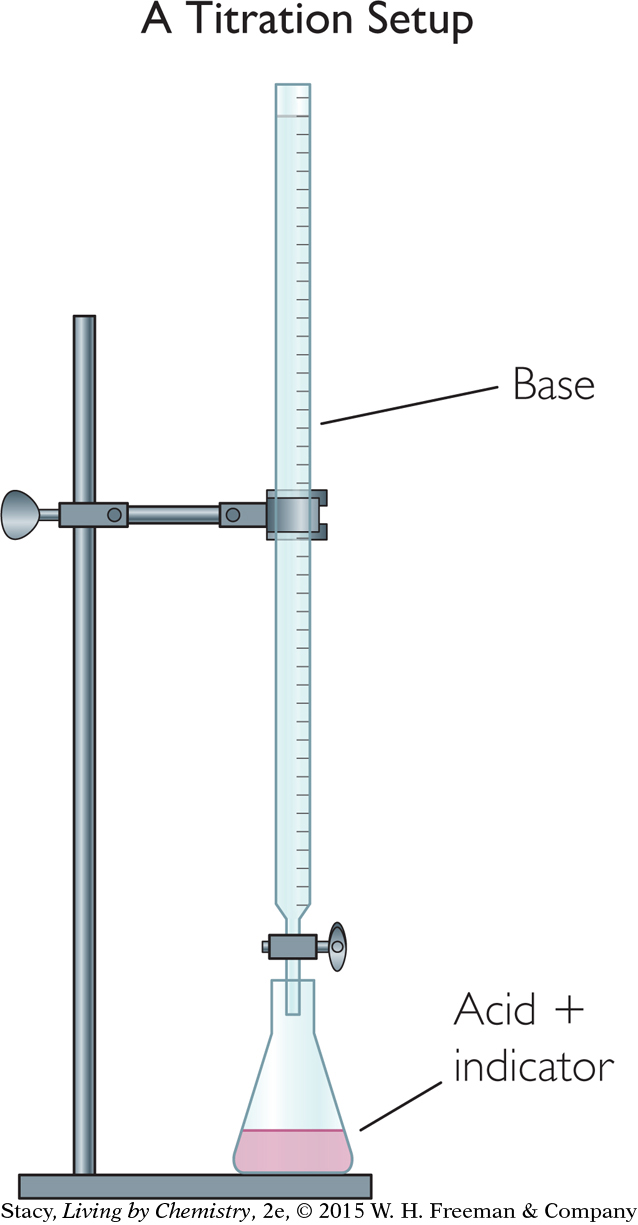
To track the spread of acid rain, scientists take water samples from lakes and test them for H+ concentration. One way to determine the concentration of a strong acid in a water sample is to use a method called a titration. A titration is a neutralization reaction that is monitored with an acid-base indicator. For example, a strong base with a known concentration is added to a strong acid sample with an unknown concentration. The base is added until the indicator changes color.

The indicator provides a visual signal that the solution has reached the equivalence point, the point at which the moles of base added have neutralized the moles of acid. By keeping track of the exact volume of base that is added to a known volume of acid, you can figure out the unknown concentration of acid in the sample.
The illustration shows a titration setup. The long thin tube is a burette. A valve on the burette allows you to regulate how much solution goes into the beaker. During a titration, solution is added from the burette until the indicator changes color. You determine the volume of solution added by noting the change in the height of the solution in the burette.
Particle Views of Titrations
Particle Views of Titrations
452
This illustration shows what happens as equal volumes of 1.0 M HCl, hydrochloric acid, and 1.0 M NaOH, sodium hydroxide, are mixed together.
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
ENVIRONMENTAL CONNECTION
ENVIRONMENTAL
CONNECTION
Acid rain creates unwanted chemical changes in soil, causing damage to trees and other plants. Around the world, acid rain and acid snow have removed calcium and magnesium from the soil and have increased levels of aluminum. When aluminum is absorbed into plants, it displaces calcium and other nutrients that the plants need.

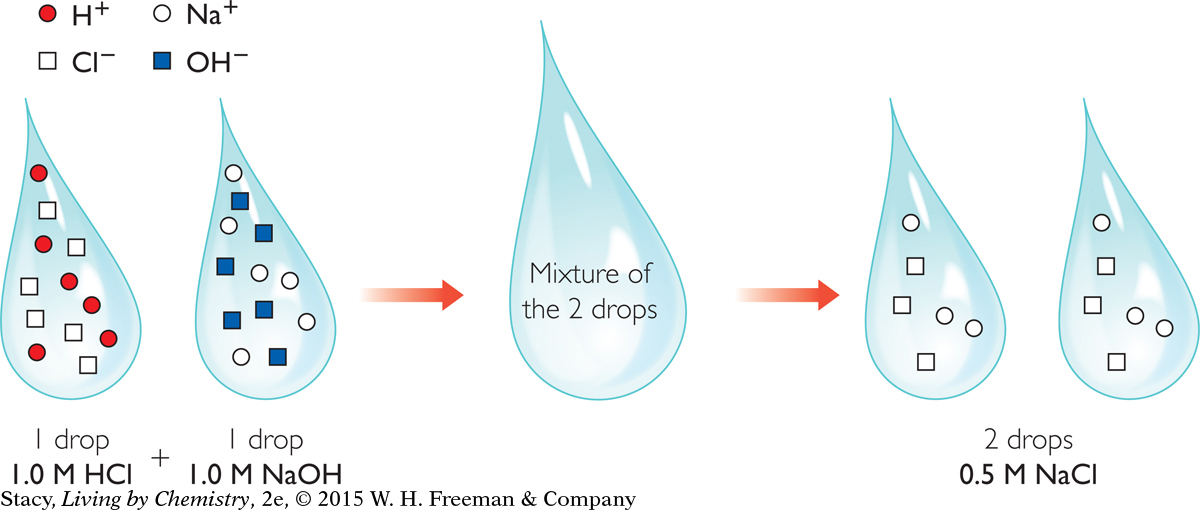
When you add one drop of 1.0 M HCl to one drop of 1.0 M NaOH, H+ ions from the acid and OH− ions from the base combine to form H2O. The Na+ ions and Cl− ions from the original drops are dissolved in the water. Notice that the concentration of Na+ ions and Cl− ions has decreased because each ion has spread out over twice the volume, equal to two drops of solution.
The next illustration shows what happens when equal volumes of 1.0 M HCl and 2.0 M NaOH are mixed together.
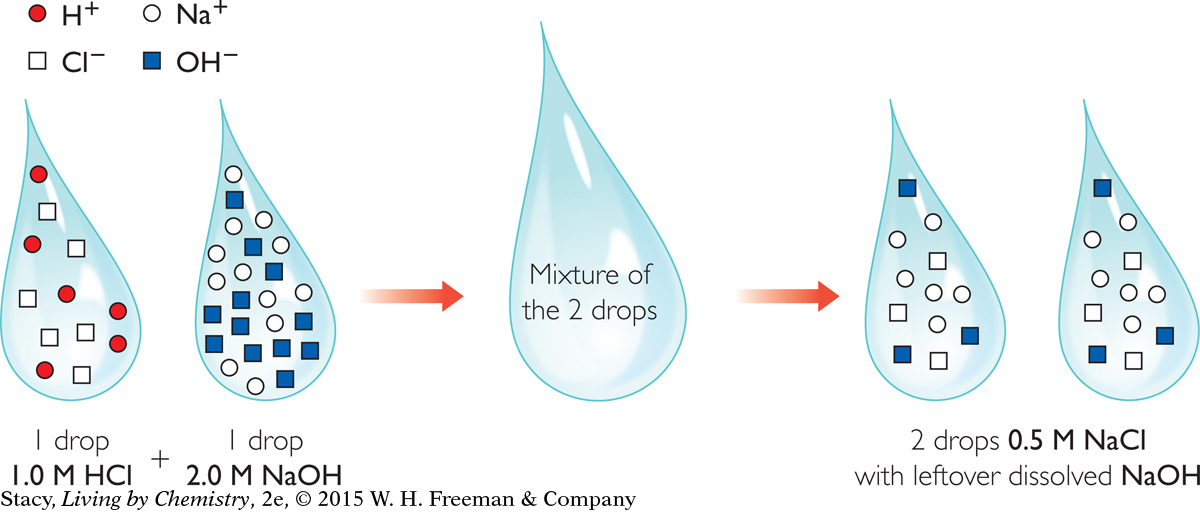
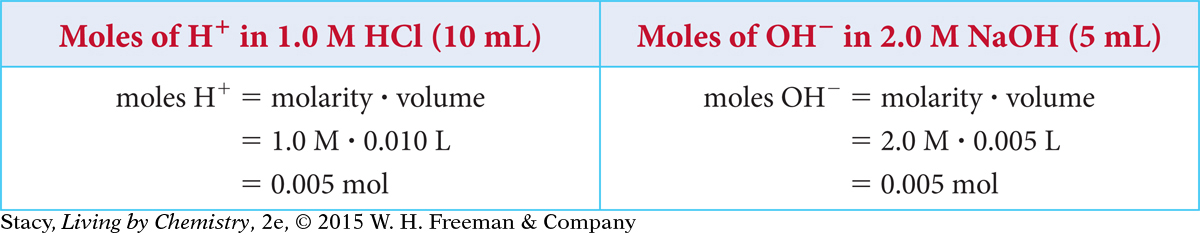
In this example, the base is more concentrated than the acid so the two-drop mixture will have leftover dissolved NaOH in addition to dissolved 0.5 M NaCl.
Because the 2.0 M NaOH is twice as concentrated as the 1.0 M HCl, you only need half as much of it to neutralize the HCl.
Titration Calculations
Titration Calculations
453

Suppose you take a 100 mL water sample from a lake contaminated with sulfuric acid, H2SO4. You want to determine the concentration of the sulfuric acid in the lake. So you titrate the sample with 2.0 molar sodium hydroxide, 2.0 M NaOH, until the solution is neutral. At the equivalence point, you know that the moles of base you added neutralized moles of acid that were in the unknown solution.
You need an indicator that changes color when the solution is neutral, so you add a drop of bromothymol blue to the lake water sample. You add NaOH slowly until the indicator turns green. Because you know the volume and molarity of the NaOH added, you can calculate the moles of acid per liter of solution in the lake water sample.
Example
Titration of H2SO4
A 100 mL sample of aqueous sulfuric acid, H2SO4, is titrated with 2.0 M NaOH. After adding 50 mL of NaOH, the indicator changes color at pH 7. What was the starting concentration of the H2SO4?
Solution
Begin by writing a balanced chemical equation.
H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + 2H2O(l)
sulfuric acid + sodium hydroxide → sodium sulfate + water
Find the total number of moles of NaOH that were used to neutralize the H2SO4.
moles NaOH = (molarity of NaOH)(volume of NaOH added)
= (2.0 mol/L)(0.050 L) = 0.10 mol NaOH
Since 2 mol of NaOH are needed to neutralize every 1 mol of H2SO4, the moles of H2SO4 in the sample must be half the number of moles of the added NaOH.
Finally, find the molarity of the sulfuric acid solution.
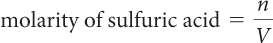
= 0.050 mol/0.100 L
= 0.50 M H2SO4
LESSON SUMMARY
LESSON SUMMARY
How can you use a neutralization reaction to figure out acid or base concentration?
KEY TERMS
titration
equivalence point
A titration is a chemical procedure carried out between an acid and a base to determine the concentration of either the acid or the base. A titration is a neutralization reaction that is monitored with an indicator. The volume of acid and the volume of base used in the procedure are carefully recorded. If the molarity of either the acid or the base is known, the molarity of the other can be determined.
454
Exercises
Reading Questions
Describe how you might use a titration to figure out the concentration of potassium hydroxide in a water sample.
What is the role of an indicator in titration?
Reason and Apply
How many mL of 0.1 M NaOH would be required to neutralize 2.0 L of 0.050 M HCl?
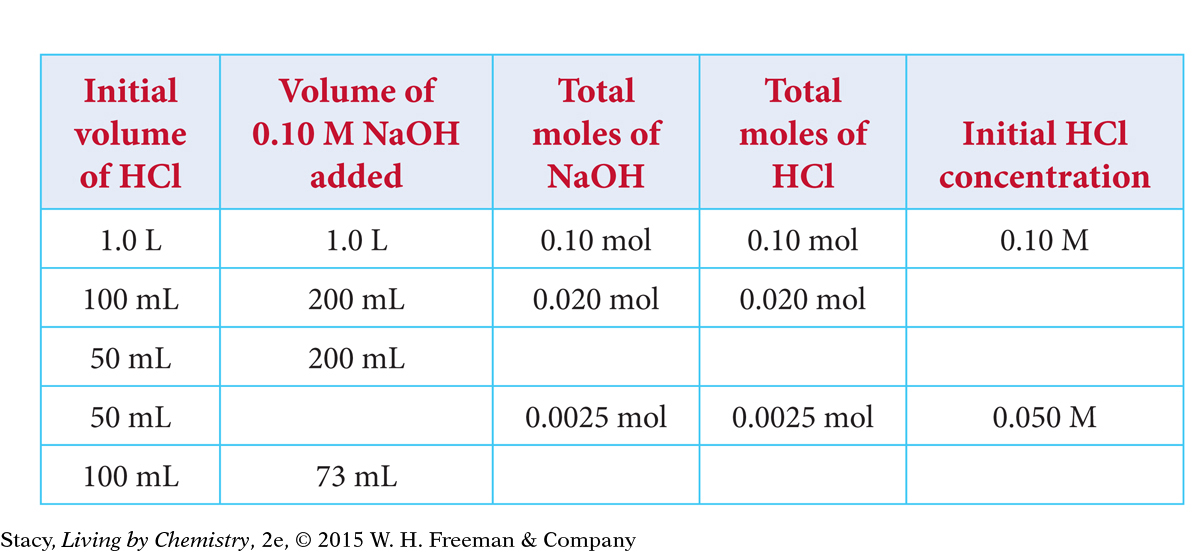
The table represents a series of titrations using 0.10 M NaOH to determine the concentrations of five different samples of HCl. Copy the table and fill in the missing values.
A student mixes 100 mL of 0.20 M HCl with different volumes of 0.50 M NaOH. Are the final solutions acidic, basic, or neutral? Explain your thinking.
100 mL of 0.20 M HCl + 20 mL of 0.50 M NaOH
100 mL of 0.20 M HCl + 40 mL of 0.50 M NaOH
100 mL of 0.20 M HCl + 60 mL of 0.50 M NaOH
Imagine that you use 0.95 M NaOH to titrate several water samples. The volume of base needed to neutralize a specified amount of acid is given. Determine the acid concentration and the pH for each.
25 mL acid, 46 mL NaOH
10 mL acid, 17 mL NaOH
25 mL acid, 12 mL NaOH