LESSON 90: Solid Evidence
THINK ABOUT IT
457
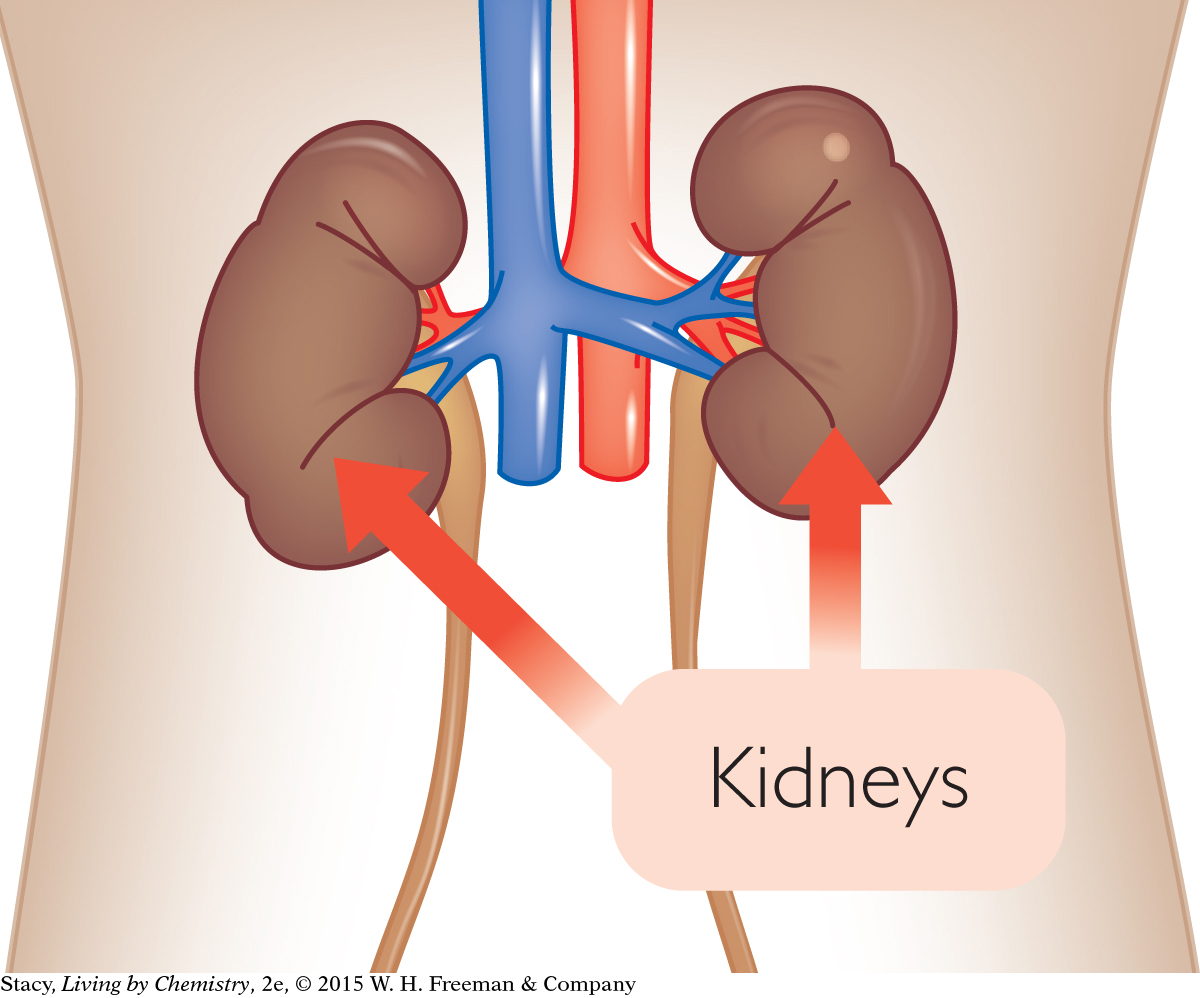
Sometimes, reactions between dissolved substances result in the formation of a solid. In your kidneys, for example, dissolved substances can react to form solid calcium oxalate. If there is enough of this solid, a painful blockage called a kidney stone can form.
Which substances precipitate from aqueous solutions?
To answer this question, you will explore
Precipitation of Ionic Solids
Solubility
Toxicity of Precipitates
Precipitation of Ionic Solids
EXPLORING THE TOPIC
Precipitation of Ionic Solids
Sometimes certain anions and cations combine and come out of solution as a solid, or a precipitate. This kind of reaction is called a precipitation reaction.
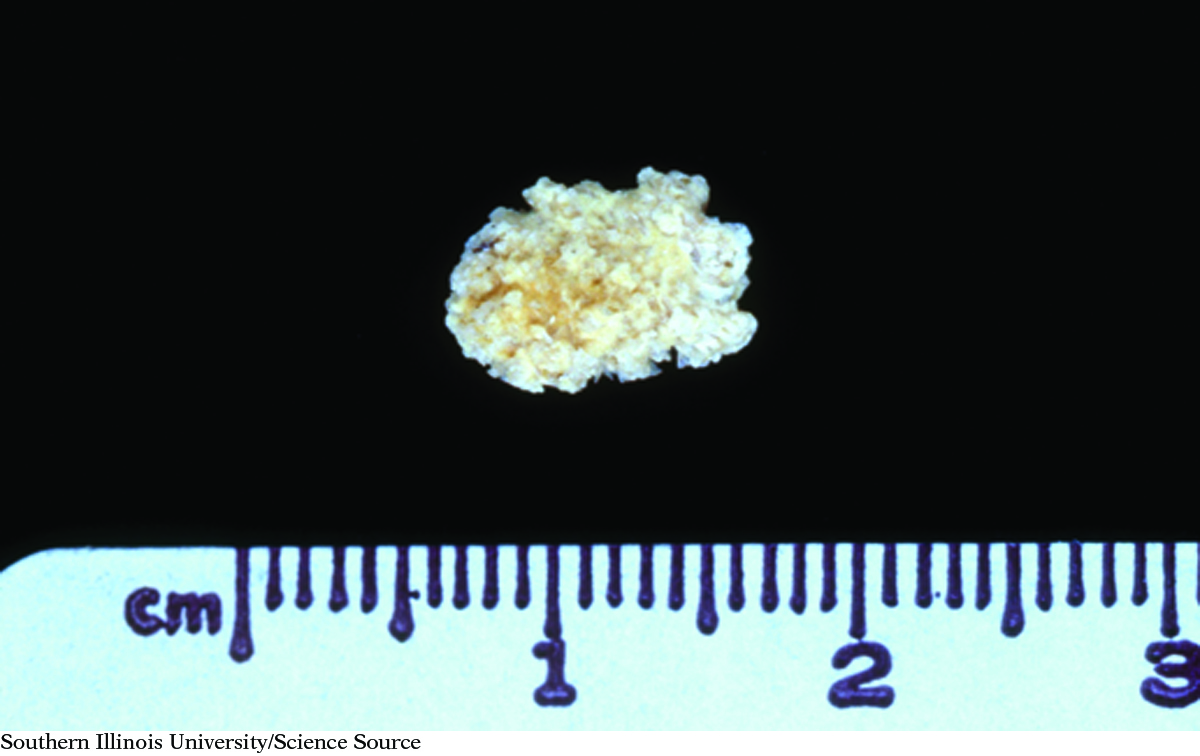
The formation of kidney stones is an example of a precipitation reaction. If the concentrations of calcium ions, Ca2+, and polyatomic oxalate ions, C2O42−, in urine become too great, calcium oxalate, CaC2O4, precipitates as kidney stones.
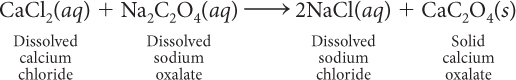
The equation for the formation of kidney stones shows that it is a double exchange reaction. Many other similar chemical combinations result in precipitation reactions.
Example 1
Precipitation of Lead Iodide
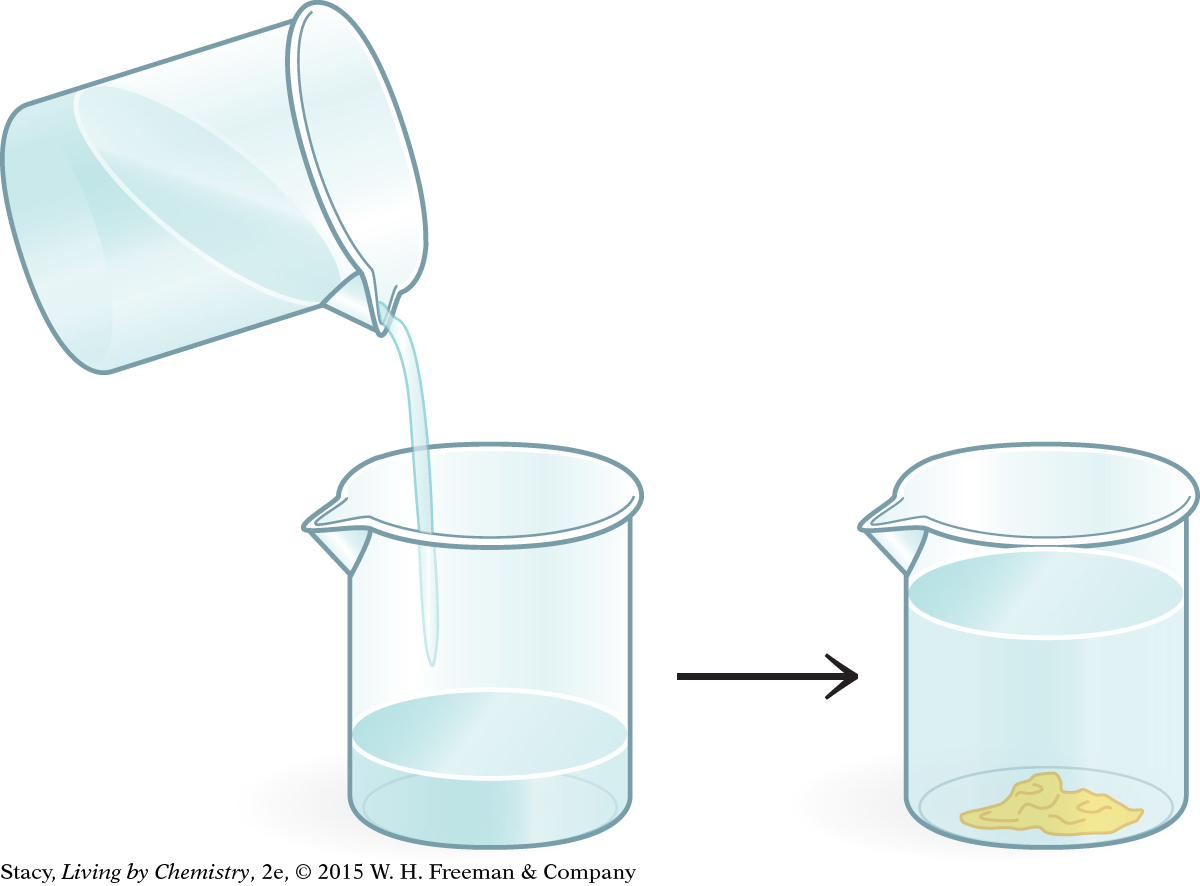
An aqueous solution of lead nitrate, Pb(NO3)2(aq), is mixed with an aqueous solution of potassium iodide, KI(aq). The result is a bright yellow solid, lead iodide, PbI2(s), in a clear solution. Write a balanced chemical equation for this precipitation reaction.
458
Solution
This is a double exchange reaction. The lead and potassium cations exchange anions with one another. Begin by writing an equation for what you know.
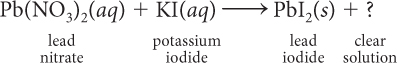
The potassium ions, K+, and the nitrate ions, NO3−, combine in a 1:1 ratio to form KNO3.
Balance the equation.
Solubility
Solubility
Ionic substances vary significantly as to how much they will dissolve in water. For some ionic substances, large quantities dissolve in water. These substances have a high solubility. For other ionic substances, only very small quantities dissolve. These substances have a low solubility. Substances with a low solubility tend to form precipitates in aqueous solutions.
Big Idea
Big Idea
Some ionic solids are more soluble than others. When a compound reaches the limits of its solubility, undissolved solid is visible.
You can use a solubility table such as the one shown here to determine the solubility of various ionic compounds. To use the table, combine a cation from the rows on the left with an anion from the columns on the right to determine if the compound formed is either very soluble (S), or insoluble/not very soluble (N).
WEATHER CONNECTION
WEATHER
CONNECTION
Precipitation can refer to either water coming out of the atmosphere as rain or snow or as compounds coming out of an aqueous solution as solids.
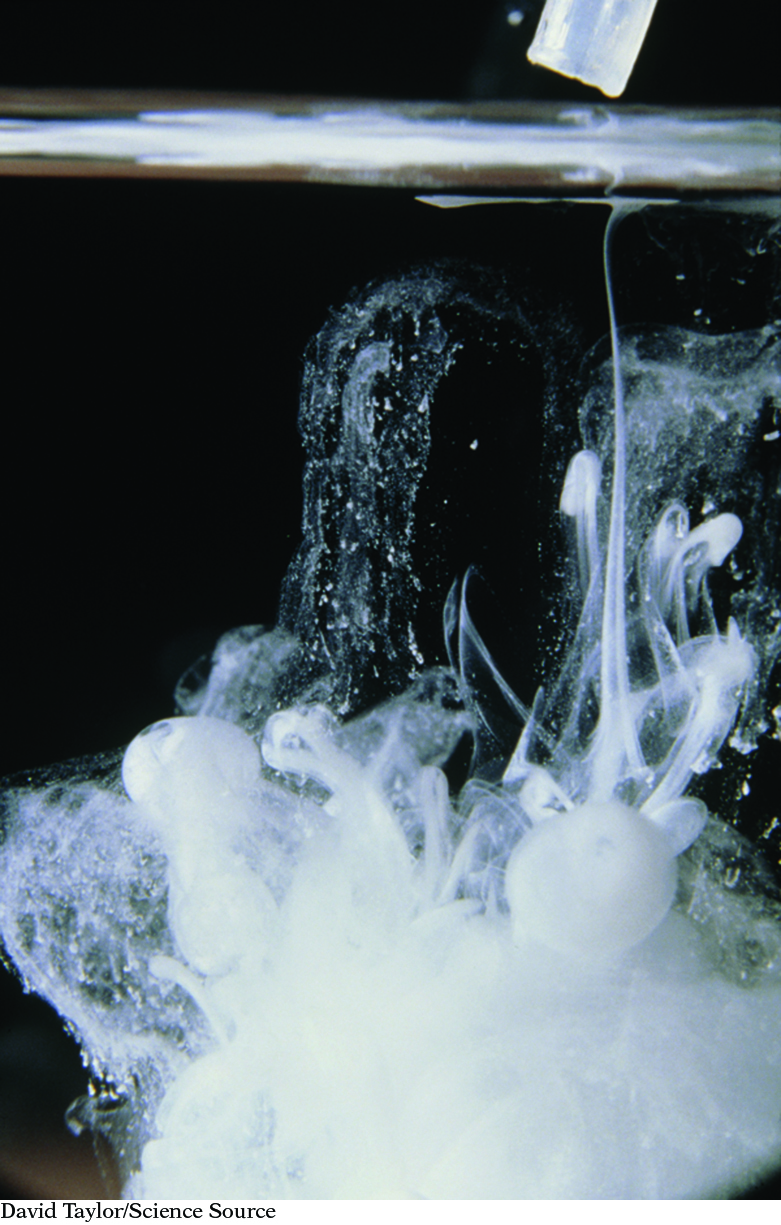
459
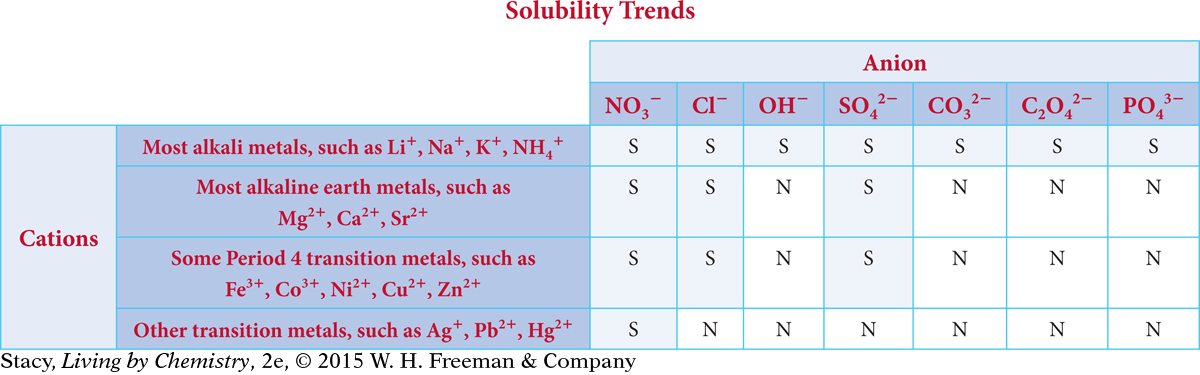
Recall that in Unit 1: Alchemy, you characterized ionic solids as soluble in water. This is true for many ionic solids. But as you can see from the solubility table, some ionic solids are not very soluble.
Example 2
Predicting Solid Products
Suppose that you combine aqueous calcium nitrate, Ca(NO3)2, with aqueous sodium phosphate, Na3PO4, and there is a double exchange reaction. Do you expect a precipitate to form?
Solution
First, write a balanced chemical equation for this reaction. You know that the two aqueous cations exchange anions:
3Ca(NO3)2(aq) + 2Na3PO4(aq) → Ca3(PO4)2(?) + 6NaNO3(?)
Next, use the solubility table to determine if there is a precipitate. According to the table, NaNO3 is soluble in water, so it remains dissolved. Ca3(PO4)2 is not very soluble, so it forms a precipitate.
3Ca(NO3)2(aq) + 2Na3PO4(aq) → Ca3(PO4)2(s) + 6NaNO3(aq)
EARTH SCIENCE CONNECTION
EARTH SCIENCE
CONNECTION
Stalactites and stalagmites are caused by precipitation of solids from water that drips from cave walls. The word stalagmite comes from the Greek word for “drip.” These beautiful cave structures are often formed from calcium carbonate.

Reactions in aqueous solutions usually involve ions in solution. To describe what is happening, you can write a complete ionic equation, which shows all of the dissolved ions involved in the reaction. Examine the complete ionic equation for the reaction in Example 2.
3Ca2+(aq) + 6NO3−(aq) + 6Na+(aq) + 2PO43−(aq) →
Ca3(PO4)2(s) + 6Na+(aq) + 6NO3−(aq)
An ion that appears on both sides of a complete ionic equation and does not directly participate in the reaction is called a spectator ion. For example, Na+ and NO3− are spectator ions in this reaction. To write a more efficient equation for this reaction, you can cancel the spectator ions on each side of the equation:
460
The result is a net ionic equation that describes the reaction in terms of only the ions that are involved in the reaction:
3Ca2+(aq) + 2PO43−(aq) → Ca3(PO4)2(s)
Toxicity of Precipitates
Toxicity of Precipitates
CAREER CONNECTION
CAREER
CONNECTION
Doctors use a type of treatment called chelation therapy to treat patients who have had long-term exposure to metals like arsenic, mercury, or lead, usually in the workplace. In chelation therapy, a compound is introduced into the bloodstream of the patient. This compound bonds with heavy metals, forming water-soluble products that are then passed out of the body.
There are positive and negative aspects to the solubility of toxic substances. On the one hand, if a substance is not very soluble, it might not react with anything, and it might pass through the body relatively unnoticed. For example, some metals that can be ingested go through our bodies without causing harm. (Imagine a child swallowing a nickel.) However, many metal compounds are soluble, and once our bodies absorb them, some can do long-term damage.
LESSON SUMMARY
LESSON SUMMARY
Which substances precipitate from aqueous solutions?
KEY TERMS
precipitate
precipitation reaction
complete ionic equation
spectator ion
net ionic equation
A precipitate is a solid that forms when a reaction in an aqueous solution produces a compound that is not very soluble. Most precipitation reactions are double exchange reactions involving ionic compounds. The cations exchange anions in solution, and one of the substances precipitates as a solid. Precipitation depends on the solubility of a compound. Some ionic compounds are not very soluble, so they precipitate from aqueous solutions. Precipitates in your body include bones, teeth, and kidney stones.
Exercises
Reading Questions
Describe what a precipitate is and how it forms.
What does solubility have to do with precipitation from solution?
Explain what a spectator ion is.
Reason and Apply
Circle the ionic solids that are soluble in water.
LiNO3 KCl MgCl2 Ca(OH)2 RbOH CaCO3 Li2CO3 PbCl2 AgCl Name solutions you can combine that will precipitate each of the compounds listed.
CaCO3
FePO4
HgCl2
AgCl
Write balanced chemical equations for these reactions. Be sure to indicate which substances are aqueous and which are solid.
NaCl(aq) + Hg(NO3)2(aq)
NaOH(aq) + CaCl2(aq)
K2CO3(aq) + LiNO3(aq)
Zn(NO3)2(aq) + Na3PO4(aq)
NaCl(aq) + Ca(NO3)2(aq)
Refer to your answers for Exercise 6. For each equation that represents a precipitation reaction, write both complete and net ionic equations.